| << Chapter < Page | Chapter >> Page > |
Even exergonic, energy-releasing reactions require a small amount of activation energy in order to proceed. However, consider endergonic reactions, which require much more energy input, because their products have more free energy than their reactants. Within the cell, where does energy to power such reactions come from? The answer lies with an energy-supplying molecule called adenosine triphosphate , or ATP . ATP is a small, relatively simple molecule ( [link] ), but within some of its bonds, it contains the potential for a quick burst of energy that can be harnessed to perform cellular work. This molecule can be thought of as the primary energy currency of cells in much the same way that money is the currency that people exchange for things they need. ATP is used to power the majority of energy-requiring cellular reactions.
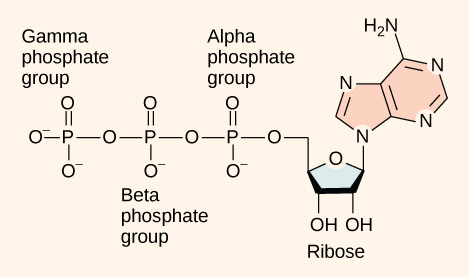
As its name suggests, adenosine triphosphate is comprised of adenosine bound to three phosphate groups ( [link] ). Adenosine is a RNA nucleotide without the phosphate (so just the nitrogenous base and sugar). Together, the adenosine and the three phosphates constitute an energy powerhouse. The bonds between the phosphates are considered "high-energy" bonds because the products of such bond breaking—adenosine diphosphate (ADP) and one inorganic phosphate group (P i )—have considerably lower free energy than the reactants: ATP and a water molecule. Because this reaction takes place with the use of a water molecule, it is considered a hydrolysis reaction. In other words, ATP is hydrolyzed into ADP in the following reaction:
Like most chemical reactions, the hydrolysis of ATP to ADP is reversible. The reverse reaction regenerates ATP from ADP + P i . Indeed, cells rely on the regeneration of ATP just as people rely on the regeneration of spent money through some sort of income. Since ATP hydrolysis releases energy, ATP regeneration must require an input of free energy. The formation of ATP is expressed in this equation:
ATP is a highly unstable molecule. Unless quickly used to perform work, ATP spontaneously dissociates into ADP + P i , and the free energy released during this process is lost as heat. So how is energy released by ATP hydrolysis used to perform work inside the cell? It depends on a strategy called energy coupling. Cells couple the exergonic reaction of ATP hydrolysis with endergonic reactions, allowing them to proceed.
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to a ribose molecule and to a single phosphate group ( [link] ). Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine di phosphate (ADP); the addition of a third phosphate group forms adenosine tri phosphate (ATP).

Notification Switch
Would you like to follow the 'General biology part i - mixed majors' conversation and receive update notifications?