| << Chapter < Page | Chapter >> Page > |
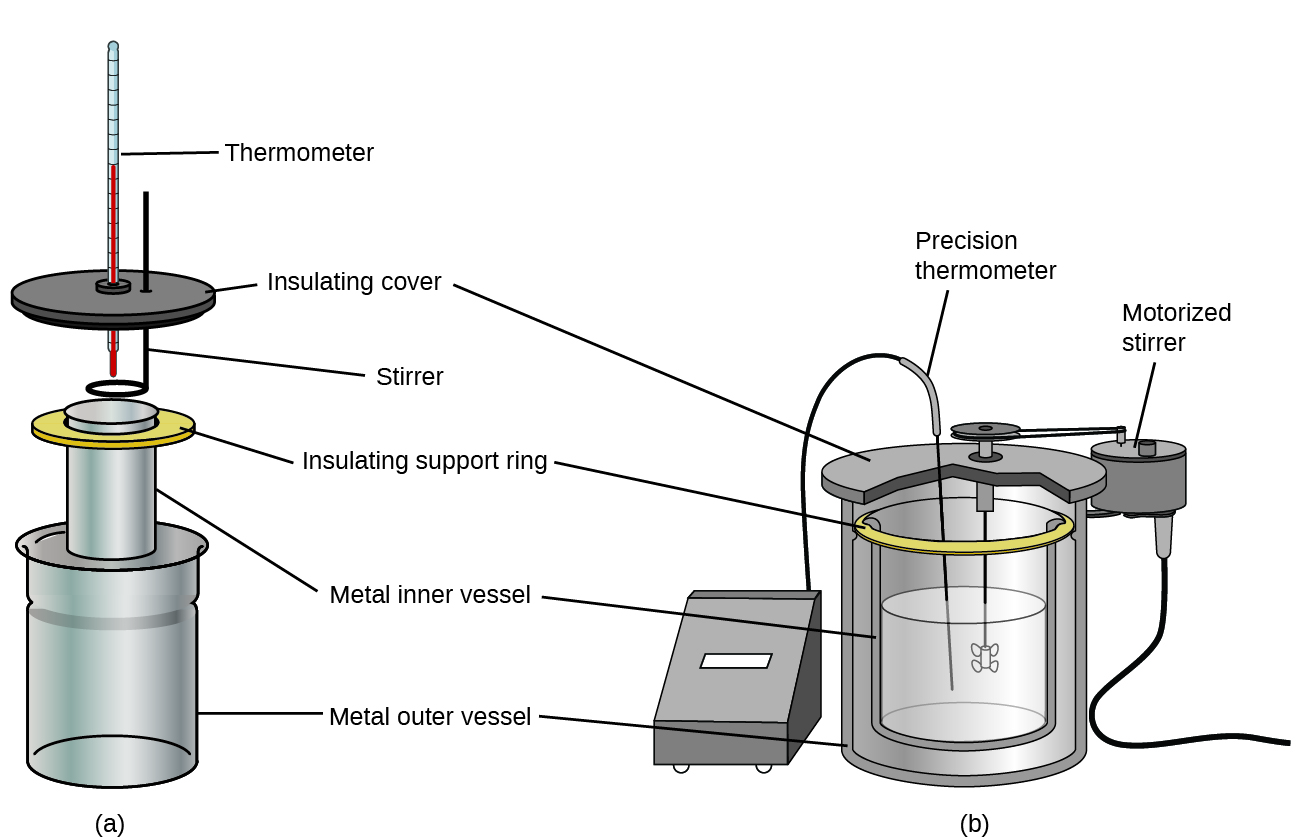
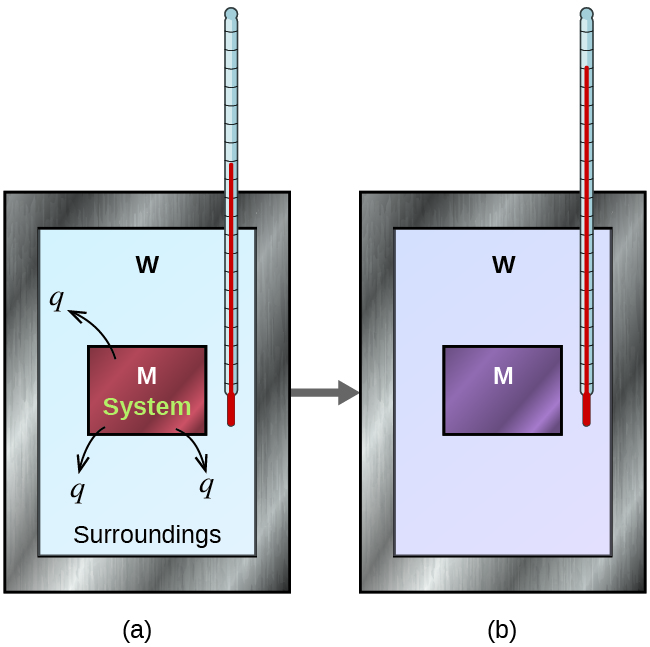
Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high-temperature substance, such as a hot piece of metal (M), and a low-temperature substance, such as cool water (W). If we place the metal in the water, heat will flow from M to W. The temperature of M will decrease, and the temperature of W will increase, until the two substances have the same temperature—that is, when they reach thermal equilibrium ( [link] ). If this occurs in a calorimeter, ideally all of this heat transfer occurs between the two substances, with no heat gained or lost by either the calorimeter or the calorimeter’s surroundings. Under these ideal circumstances, the net heat change is zero:
This relationship can be rearranged to show that the heat gained by substance M is equal to the heat lost by substance W:
The magnitude of the heat (change) is therefore the same for both substances, and the negative sign merely shows that q substance M and q substance W are opposite in direction of heat flow (gain or loss) but does not indicate the arithmetic sign of either q value (that is determined by whether the matter in question gains or loses heat, per definition). In the specific situation described, q substance M is a negative value and q substance W is positive, since heat is transferred from M to W.
Since we know how heat is related to other measurable quantities, we have:
Letting f = final and i = initial, in expanded form, this becomes:
The density of water is 1.0 g/mL, so 425 mL of water = 425 g. Noting that the final temperature of both the rebar and water is 42.7 °C, substituting known values yields:

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?