| << Chapter < Page | Chapter >> Page > |
Define the following and give an example of each:
(a) dispersion force
(b) dipole-dipole attraction
(c) hydrogen bond
(a) Dispersion forces occur as an atom develops a temporary dipole moment when its electrons are distributed asymmetrically about the nucleus. This structure is more prevalent in large atoms such as argon or radon. A second atom can then be distorted by the appearance of the dipole in the first atom. The electrons of the second atom are attracted toward the positive end of the first atom, which sets up a dipole in the second atom. The net result is rapidly fluctuating, temporary dipoles that attract one another (example: Ar). (b) A dipole-dipole attraction is a force that results from an electrostatic attraction of the positive end of one polar molecule for the negative end of another polar molecule (example: ICI molecules attract one another by dipole-dipole interaction). (c) Hydrogen bonds form whenever a hydrogen atom is bonded to one of the more electronegative atoms, such as a fluorine, oxygen, or nitrogen atom. The electrostatic attraction between the partially positive hydrogen atom in one molecule and the partially negative atom in another molecule gives rise to a strong dipole-dipole interaction called a hydrogen bond (example:
The types of intermolecular forces in a substance are identical whether it is a solid, a liquid, or a gas. Why then does a substance change phase from a gas to a liquid or to a solid?
Why do the boiling points of the noble gases increase in the order He<Ne<Ar<Kr<Xe?
The London forces typically increase as the number of electrons increase.
Neon and HF have approximately the same molecular masses.
(a) Explain why the boiling points of Neon and HF differ.
(b) Compare the change in the boiling points of Ne, Ar, Kr, and Xe with the change of the boiling points of HF, HCl, HBr, and HI, and explain the difference between the changes with increasing atomic or molecular mass.
Arrange each of the following sets of compounds in order of increasing boiling point temperature:
(a) HCl, H 2 O, SiH 4
(b) F 2 , Cl 2 , Br 2
(c) CH 4 , C 2 H 6 , C 3 H 8
(d) O 2 , NO, N 2
(a) SiH 4 <HCl<H 2 O; (b) F 2 <Cl 2 <Br 2 ; (c) CH 4 <C 2 H 6 <C 3 H 8 ; (d) N 2 <O 2 <NO
The molecular mass of butanol, C 4 H 9 OH, is 74.14; that of ethylene glycol, CH 2 (OH)CH 2 OH, is 62.08, yet their boiling points are 117.2 °C and 174 °C, respectively. Explain the reason for the difference.
On the basis of intermolecular attractions, explain the differences in the boiling points of n –butane (−1 °C) and chloroethane (12 °C), which have similar molar masses.
Only rather small dipole-dipole interactions from C-H bonds are available to hold n -butane in the liquid state. Chloroethane, however, has rather large dipole interactions because of the Cl-C bond; the interaction is therefore stronger, leading to a higher boiling point.
On the basis of dipole moments and/or hydrogen bonding, explain in a qualitative way the differences in the boiling points of acetone (56.2 °C) and 1-propanol (97.4 °C), which have similar molar masses.
The melting point of H 2 O( s ) is 0 °C. Would you expect the melting point of H 2 S( s ) to be −85 °C, 0 °C, or 185 °C? Explain your answer.
−85 °C. Water has stronger hydrogen bonds so it melts at a higher temperature.
Silane (SiH 4 ), phosphine (PH 3 ), and hydrogen sulfide (H 2 S) melt at −185 °C, −133 °C, and −85 °C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?
Explain why a hydrogen bond between two water molecules is weaker than a hydrogen bond between two hydrogen fluoride molecules.
The hydrogen bond between two hydrogen fluoride molecules is stronger than that between two water molecules because the electronegativity of F is greater than that of O. Consequently, the partial negative charge on F is greater than that on O. The hydrogen bond between the partially positive H and the larger partially negative F will be stronger than that formed between H and O.
Under certain conditions, molecules of acetic acid, CH 3 COOH, form “dimers,” pairs of acetic acid molecules held together by strong intermolecular attractions:

Draw a dimer of acetic acid, showing how two CH 3 COOH molecules are held together, and stating the type of IMF that is responsible.
Proteins are chains of amino acids that can form in a variety of arrangements, one of which is a helix. What kind of IMF is responsible for holding the protein strand in this shape? On the protein image, show the locations of the IMFs that hold the protein together:
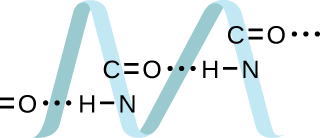
H-bonding is the principle IMF holding the DNA strands together. The H-bonding is between the and
The density of liquid NH 3 is 0.64 g/mL; the density of gaseous NH 3 at STP is 0.0007 g/mL. Explain the difference between the densities of these two phases.
Identify the intermolecular forces present in the following solids:
(a) CH 3 CH 2 OH
(b) CH 3 CH 2 CH 3
(c) CH 3 CH 2 Cl
(a) hydrogen bonding and dispersion forces; (b) dispersion forces; (c) dipole-dipole attraction and dispersion forces

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?