| << Chapter < Page | Chapter >> Page > |
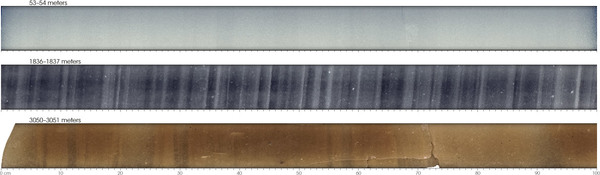
Scientists drill into these ice sheets to extract ice cores , which record information about past climates. Figure Ice Cores shows what these cores look like when they are cut open. Like tree rings, ice cores indicate years of growth. Note how the middle core (which required over a mile of drilling to extract!) has distinct layers—this is because the seasons leave an imprint in the layers of snow. Scientists can use this imprint to help calculate the age of the ice at different depths, although the task becomes more difficult the deeper the core sample, since the ice layers become more compressed. The ice records several different types of climate information: the temperature of the core, the properties of the water that make up the ice, trapped dust, and tiny entombed bubbles of ancient atmosphere.
The water molecules that make up the ice record information about the temperature of the atmosphere. Each water molecule is made up of two hydrogen atoms and one oxygen atom (and so has the chemical name H 2 O). Not all oxygen atoms are the same however; some are "light" and some are "heavy". These different types of oxygen are called isotopes , which are atoms that have same number of protons but different numbers of neutrons. The heavy isotope of oxygen (oxygen-18, or 18 O) is more than 10% heavier than the light isotope (oxygen-16 or 16 O). This means that some water molecules weigh more than others. This is important because lighter water molecules are more easily evaporated from the ocean, and once in the atmosphere, heavier water molecules are more likely to condense and fall as precipitation. As we can see from Figure Oxygen Schematic , the water in the ice sheets is lighter (has a higher proportion of 16 O relative to 18 O) than the water in the oceans.
The process of differentiation between heavy and light water molecules is temperature dependent. If the atmosphere is warm, there is more energy available to evaporate and hold the heavier 18 O water in the atmosphere, so the snow that falls on the polar ice sheets is relatively higher in 18 O. When the atmosphere is cold, the amount of energy is less, and so less 18 O makes it to the poles to be turned into glacial ice. We can compare the amount of 18 O in different parts of the ice core to see how the atmosphere's temperature—the climate—has changed.

Figure Ice Age Temperature shows what this record looks like over the last 400,000 years. The blue and green lines depict two different Antarctic ice cores (taken from ice about 350 miles apart) and the variations in oxygen isotopes are converted into temperature changes. The y-axis shows temperature change; today's climate is at zero—the dashed line. Notice that the Earth's climate has not been stable! Sometimes the temperature is higher than it is today—the blue and green lines are higher than the dashed about 120,000 years ago, for example. Most of the time the climate is much colder than today's, however: the most common value is around -6 o C (-13 o F). On average, the earth's temperature between 25,000 and 100,000 years ago was about 6 o C lower than it is today. These changes can be double-checked by measuring the temperature of the ice in the cores directly. Ice that is 30,000 years old is indeed colder than the ice made today, just as the isotope data predicts.

Notification Switch
Would you like to follow the 'Sustainability: a comprehensive foundation' conversation and receive update notifications?