| << Chapter < Page | Chapter >> Page > |
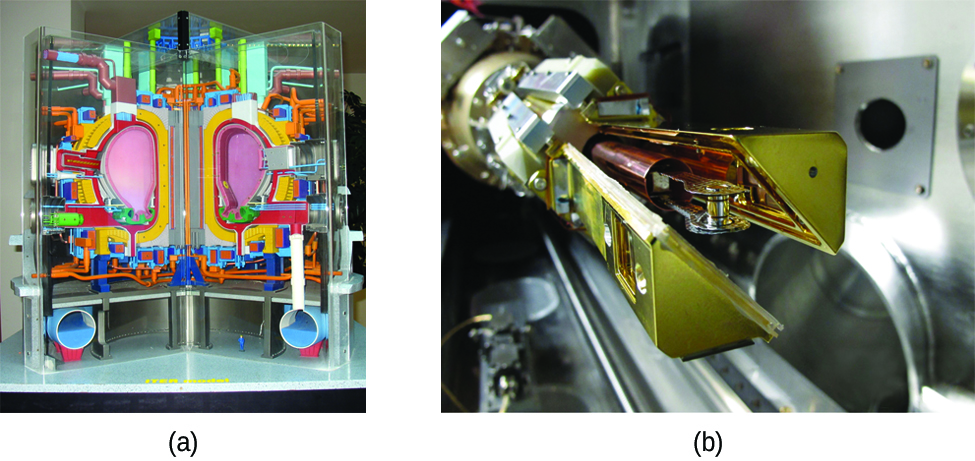
Another much more beneficial way to create fusion reactions is in a fusion reactor , a nuclear reactor in which fusion reactions of light nuclei are controlled. Because no solid materials are stable at such high temperatures, mechanical devices cannot contain the plasma in which fusion reactions occur. Two techniques to contain plasma at the density and temperature necessary for a fusion reaction are currently the focus of intensive research efforts: containment by a magnetic field and by the use of focused laser beams ( [link] ). A number of large projects are working to attain one of the biggest goals in science: getting hydrogen fuel to ignite and produce more energy than the amount supplied to achieve the extremely high temperatures and pressures that are required for fusion. At the time of this writing, there are no self-sustaining fusion reactors operating in the world, although small-scale controlled fusion reactions have been run for very brief periods.
It is possible to produce new atoms by bombarding other atoms with nuclei or high-speed particles. The products of these transmutation reactions can be stable or radioactive. A number of artificial elements, including technetium, astatine, and the transuranium elements, have been produced in this way.
Nuclear power as well as nuclear weapon detonations can be generated through fission (reactions in which a heavy nucleus is split into two or more lighter nuclei and several neutrons). Because the neutrons may induce additional fission reactions when they combine with other heavy nuclei, a chain reaction can result. Useful power is obtained if the fission process is carried out in a nuclear reactor. The conversion of light nuclei into heavier nuclei (fusion) also produces energy. At present, this energy has not been contained adequately and is too expensive to be feasible for commercial energy production.
Write the balanced nuclear equation for the production of the following transuranium elements:
(a) berkelium-244, made by the reaction of Am-241 and He-4
(b) fermium-254, made by the reaction of Pu-239 with a large number of neutrons
(c) lawrencium-257, made by the reaction of Cf-250 and B-11
(d) dubnium-260, made by the reaction of Cf-249 and N-15
(a) (b) (c) (d)
How does nuclear fission differ from nuclear fusion? Why are both of these processes exothermic?
Both fusion and fission are nuclear reactions. Why is a very high temperature required for fusion, but not for fission?
Two nuclei must collide for fusion to occur. High temperatures are required to give the nuclei enough kinetic energy to overcome the very strong repulsion resulting from their positive charges.
Cite the conditions necessary for a nuclear chain reaction to take place. Explain how it can be controlled to produce energy, but not produce an explosion.
Describe the components of a nuclear reactor.
A nuclear reactor consists of the following:
1. A nuclear fuel. A fissionable isotope must be present in large enough quantities to sustain a controlled chain reaction. The radioactive isotope is contained in tubes called fuel rods.
2. A moderator. A moderator slows neutrons produced by nuclear reactions so that they can be absorbed by the fuel and cause additional nuclear reactions.
3. A coolant. The coolant carries heat from the fission reaction to an external boiler and turbine where it is transformed into electricity.
4. A control system. The control system consists of control rods placed between fuel rods to absorb neutrons and is used to adjust the number of neutrons and keep the rate of the chain reaction at a safe level.
5. A shield and containment system. The function of this component is to protect workers from radiation produced by the nuclear reactions and to withstand the high pressures resulting from high-temperature reactions.
In usual practice, both a moderator and control rods are necessary to operate a nuclear chain reaction safely for the purpose of energy production. Cite the function of each and explain why both are necessary.
Describe how the potential energy of uranium is converted into electrical energy in a nuclear power plant.
The fission of uranium generates heat, which is carried to an external steam generator (boiler). The resulting steam turns a turbine that powers an electrical generator.
The mass of a hydrogen atom is 1.007825 amu; that of a tritium atom is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of produced is released by the following fusion reaction:

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?