| << Chapter < Page | Chapter >> Page > |
Corrosion is usually defined as the degradation of metals due to an electrochemical process. The formation of rust on iron, tarnish on silver, and the blue-green patina that develops on copper are all examples of corrosion. The total cost of corrosion in the United States is significant, with estimates in excess of half a trillion dollars a year.
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin. The formation of the protective layer is a form of passivation, which is discussed further in a later chapter.
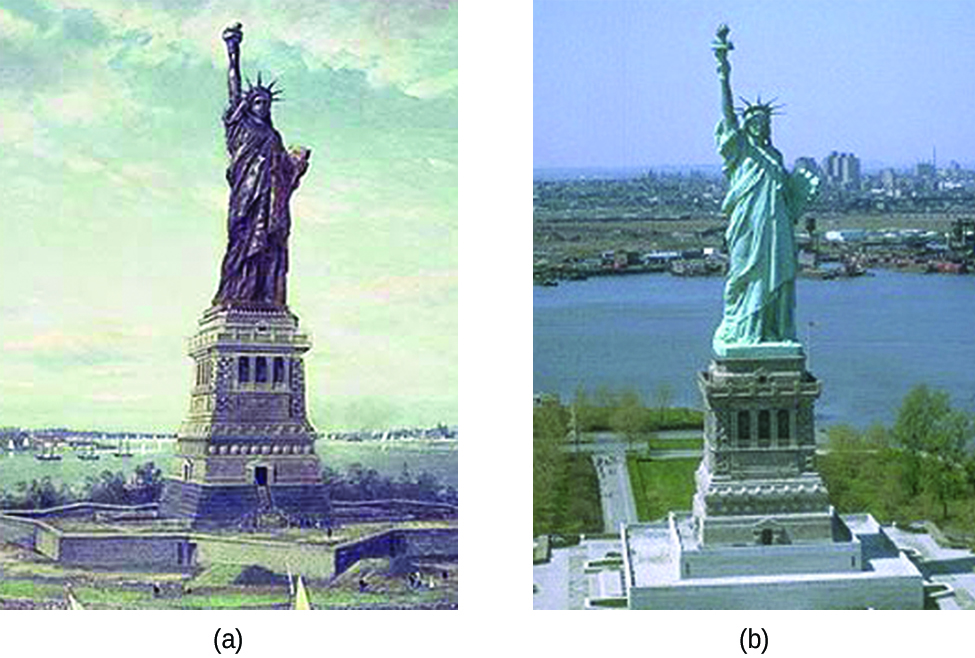
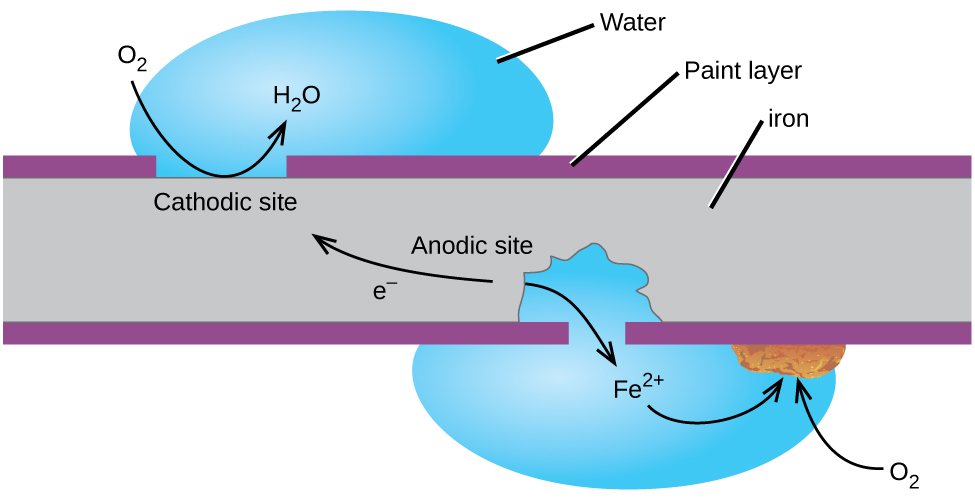
Perhaps the most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following ( [link] ). Once exposed to the atmosphere, iron rapidly oxidizes.
The electrons reduce oxygen in the air in acidic solutions.
What we call rust is hydrated iron(III) oxide, which forms when iron(II) ions react further with oxygen.
The number of water molecules is variable, so it is represented by x . Unlike the patina on copper, the formation of rust does not create a protective layer and so corrosion of the iron continues as the rust flakes off and exposes fresh iron to the atmosphere.

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?