| << Chapter < Page | Chapter >> Page > |
The second type of beta decay is less common than the first. It is
decay. Certain nuclides decay by the emission of a
positive electron. This is
antielectron or
positron decay (see
[link] ).
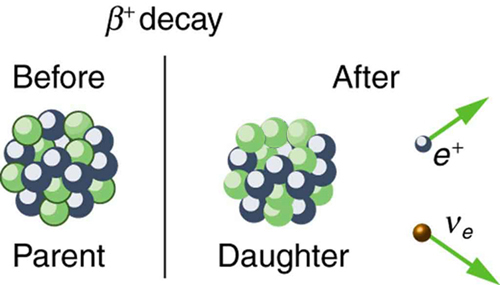
The antielectron is often represented by the symbol , but in beta decay it is written as to indicate the antielectron was emitted in a nuclear decay. Antielectrons are the antimatter counterpart to electrons, being nearly identical, having the same mass, spin, and so on, but having a positive charge and an electron family number of . When a positron encounters an electron, there is a mutual annihilation in which all the mass of the antielectron-electron pair is converted into pure photon energy. (The reaction, , conserves electron family number as well as all other conserved quantities.) If a nuclide is known to decay, then its decay equation is
where Y is the nuclide having one less proton than X (to conserve charge) and is the symbol for the electron’s neutrino , which has an electron family number of . Since an antimatter member of the electron family (the ) is created in the decay, a matter member of the family (here the ) must also be created. Given, for example, that decays, you can write its full decay equation by first finding that for , so that the daughter nuclide will have , the atomic number for neon. Thus the decay equation for is
In decay, it is as if one of the protons in the parent nucleus decays into a neutron, a positron, and a neutrino. Protons do not do this outside of the nucleus, and so the decay is due to the complexities of the nuclear force. Note again that the total number of nucleons is constant in this and any other reaction. To find the energy emitted in decay, you must again count the number of electrons in the neutral atoms, since atomic masses are used. The daughter has one less electron than the parent, and one electron mass is created in the decay. Thus, in decay,
since we use the masses of neutral atoms.
Electron capture is the third type of beta decay. Here, a nucleus captures an inner-shell electron and undergoes a nuclear reaction that has the same effect as decay. Electron capture is sometimes denoted by the letters EC. We know that electrons cannot reside in the nucleus, but this is a nuclear reaction that consumes the electron and occurs spontaneously only when the products have less mass than the parent plus the electron. If a nuclide is known to undergo electron capture, then its electron capture equation is
Any nuclide that can decay can also undergo electron capture (and often does both). The same conservation laws are obeyed for EC as for decay. It is good practice to confirm these for yourself.
All forms of beta decay occur because the parent nuclide is unstable and lies outside the region of stability in the chart of nuclides. Those nuclides that have relatively more neutrons than those in the region of stability will decay to produce a daughter with fewer neutrons, producing a daughter nearer the region of stability. Similarly, those nuclides having relatively more protons than those in the region of stability will decay or undergo electron capture to produce a daughter with fewer protons, nearer the region of stability.

Notification Switch
Would you like to follow the 'Concepts of physics' conversation and receive update notifications?