| << Chapter < Page | Chapter >> Page > |
Bromine trifluoride (BrF 3 ) has a liquid range similar to water (Mp = 8.8 °C and Bp = 127 °C), and like water it auto ionizes, [link] .
The products, like those of water’s self-ionization, are an acid (BrF 2 + ) and a base (BrF 4 - ). However, unlike water, BrF 3 reacts with fluoride acids and bases not proton acids and bases. Thus, in BrF 3 a base is a salt that provides F - , i.e., potassium fluoride (KF) is a base in BrF 3 solution in the same manner as potassium hydroxide (KOH) is a base in water. The product from the reaction of a fluoride donor salt with BrF 3 is the formation of the conjugate base, BrF 4 - , [link] .
Other examples of this type of reaction include:
By analogy, an acid in BrF 3 solution is a compound that acts as a fluoride (F - ) acceptor, i.e., a Lewis acid, [link] .
Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., VF 5 ). A wide range of salts and oxides may be converted to fluorides with the metal in a high oxidation state. However, it should be noted that BeO, MgO, and Al 2 O 3 form oxo fluorides rather than the fluoride.
The reaction of silver with BrF 3 yields the monofluoride, while the same reaction with gold yields the trifluoride, Eq. If the reactions are combined in BrF 3 solution a mixed metal fluoride salt is formed, [link] .
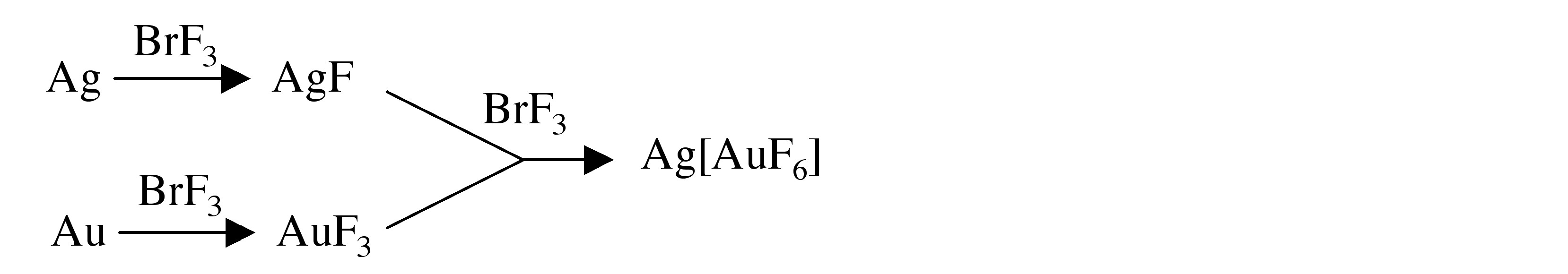
A similar reaction occurs with NOCl and V 2 O 5 , [link] .

What are the products from the reaction of BrF 3 with (a) Sb 2 O 5 , (b) KCl, and (c) a mixture of Sb 2 O 5 and KCl?
(a) SbF 5 , (b) KF, and (c) K[SbF 6 ].

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?