| << Chapter < Page | Chapter >> Page > |
We can also do variations of this type of calculation, as shown in the next example.
If we let x represent the fraction that is 35 Cl, then the fraction that is 37 Cl is represented by 1.00 − x .
(The fraction that is 35 Cl + the fraction that is 37 Cl must add up to 1, so the fraction of 37 Cl must equal 1.00 − the fraction of 35 Cl.)
Substituting this into the average mass equation, we have:
So solving yields: x = 0.7576, which means that 1.00 − 0.7576 = 0.2424. Therefore, chlorine consists of 75.76% 35 Cl and 24.24% 37 Cl.
69.15% Cu-63 and 30.85% Cu-65
Visit this site to make mixtures of the main isotopes of the first 18 elements, gain experience with average atomic mass, and check naturally occurring isotope ratios using the Isotopes and Atomic Mass simulation.
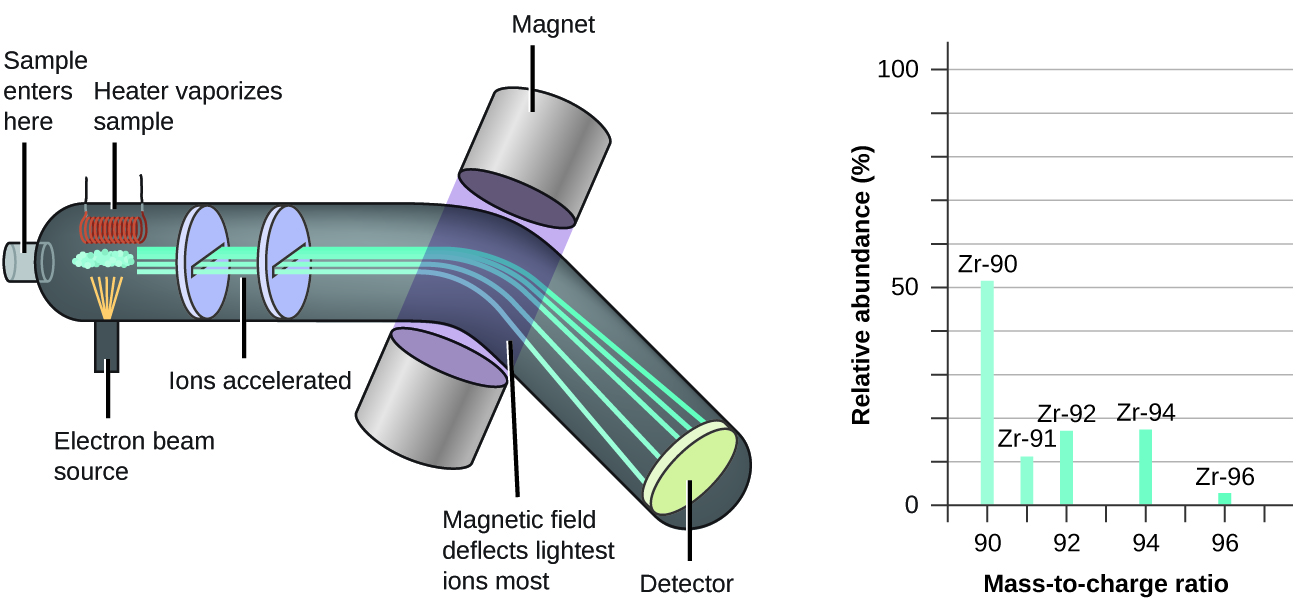
The occurrence and natural abundances of isotopes can be experimentally determined using an instrument called a mass spectrometer. Mass spectrometry (MS) is widely used in chemistry, forensics, medicine, environmental science, and many other fields to analyze and help identify the substances in a sample of material. In a typical mass spectrometer ( [link] ), the sample is vaporized and exposed to a high-energy electron beam that causes the sample’s atoms (or molecules) to become electrically charged, typically by losing one or more electrons. These cations then pass through a (variable) electric or magnetic field that deflects each cation’s path to an extent that depends on both its mass and charge (similar to how the path of a large steel ball bearing rolling past a magnet is deflected to a lesser extent that that of a small steel BB). The ions are detected, and a plot of the relative number of ions generated versus their mass-to-charge ratios (a mass spectrum ) is made. The height of each vertical feature or peak in a mass spectrum is proportional to the fraction of cations with the specified mass-to-charge ratio. Since its initial use during the development of modern atomic theory, MS has evolved to become a powerful tool for chemical analysis in a wide range of applications.

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?