| << Chapter < Page | Chapter >> Page > |
| Element | Relative atomic mass (u) | Molar mass ( ) | Mass of one mole of the element (g) |
| Magnesium | 24,31 | 24,31 | 24,31 |
| Lithium | 6,94 | 6,94 | 6,94 |
| Oxygen | 16 | 16 | 16 |
| Nitrogen | 14,01 | 14,01 | 14,01 |
| Iron | 55,85 | 55,85 | 55,85 |
Calculate the number of moles of iron ( ) in a sample.
If we look at the periodic table, we see that the molar mass of iron is . This means that 1 mole of iron will have a mass of .
If 1 mole of iron has a mass of , then: the number of moles of iron in must be:
There are 2 moles of iron in the sample.
You have a sample that contains 5 moles of zinc.
Molar mass of zinc is , meaning that 1 mole of zinc has a mass of .
If 1 mole of zinc has a mass of , then 5 moles of zinc has a mass of: (answer to a)
(answer to b)
The calculations that have been used so far, can be made much simpler by using the following equation:
The equation can also be used to calculate mass and molar mass, using the following equations:
and
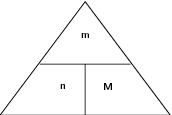
The following diagram may help to remember the relationship between these three variables. You need to imagine that the horizontal line is like a 'division' sign and that the vertical line is like a 'multiplication' sign. So, for example, if you want to calculate 'M', then the remaining two letters in the triangle are 'm' and 'n' and 'm' is above 'n' with a division sign between them. In your calculation then, 'm' will be the numerator and 'n' will be the denominator.
Calculate the number of moles of copper there are in a sample that weighs .
There are 2 moles of copper in the sample.
You are given a 5 mol sample of sodium. What mass of sodium is in the sample?
M
Therefore,
The sample of sodium has a mass of .

Notification Switch
Would you like to follow the 'Chemistry grade 10 [caps]' conversation and receive update notifications?