| << Chapter < Page | Chapter >> Page > |
This argument would seem to apply perfectly well to an electron in either the 2s or 2p orbital, since both are in the n = 2 shell. So this does not account for the difference in the energies of these orbitals. It does account for the similarity of the energies of these orbitals, as clearly seen in the data in [link] . But we still need to know why there is a difference.
The answer lies in remembering that in each orbital we only know the probabilities for where the electrons might be observed. Therefore, we cannot definitely state that the n = 2 electrons are “outside” of the 1s core electrons; we can only say that they probably are. As a result, the shielding effect is not perfect. In fact, there is some probability that an n = 2 electron might be found closer to the nucleus than the 1s electrons. This is called “core penetration.” When an n = 2 electron does penetrate the core, it is no longer shielded from the nucleus. In this case, the n = 2 electron is very strongly attracted to the nucleus and its energy is thus lowered. Therefore, the effect of core penetration is to reduce the shielding effect and therefore increase the effective nuclear charge.
What is the extent of this penetration? The answer is in [link] , which shows the probability of finding an electron a distance r away from the nucleus for each of the 1s, 2s, and 2p orbitals. If we compare the probabilities, we see that there is a slightly greater probability (though still quite small) for the 2s electron to penetrate the core than for the 2p electron to do so.
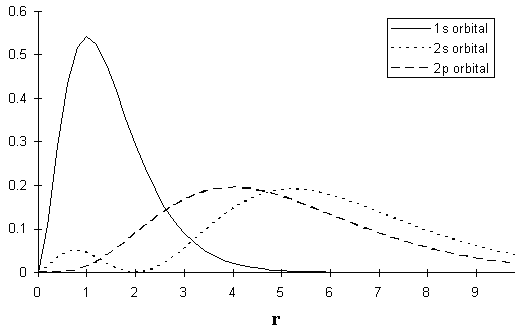
As a result of the core penetration, an electron in a 2s orbital feels a greater “effective nuclear charge” than just the core charge, which was approximated by assuming perfect shielding. Thus, the effective nuclear charge for a 2s electron is greater than the effective nuclear charge for a 2p electron. Therefore, the energy of an electron in the 2s orbital in beryllium is lower than it would be in the 2p orbital.
Knowing how the electrons move about the nucleus explains the ionization energy data in [link] and also explains the shell model of the atom by explaining why each shell can hold a limited number of electrons. This means that we can understand the Periodic Law and the trends in the Periodic Table using this new model. It turns out that we can also use our understanding of the motions of the electrons in atoms to help understand how and why atoms bond together to form molecules. This is a very important question for chemists, because understanding bonding is the key to understanding how and why molecules react with each other. Our understanding of electron orbitals will be very useful in answering these questions.
By John S. Hutchinson, Rice University, 2011

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?