| << Chapter < Page | Chapter >> Page > |
Represent the molecule (hydrogen cyanide) using Lewis notation
The electron configuration of hydrogen is , the electron configuration of nitrogen is and for carbon is . This means that hydrogen has one valence electron which is unpaired, carbon has four valence electrons, all of which are unpaired, and nitrogen has five valence electrons, three of which are unpaired.
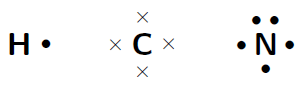
The molecule is represented below. Notice the three electron pairs between the nitrogen and carbon atom. Because these three covalent bonds are between the same two atoms, this is a triple bond.

Represent the molecule (hydrogen sulphide) using Lewis notation
Hydrogen has an electron configuration of and sulphur has an electron configuration of . Each hydrogen atom has one valence electron which is unpaired, and sulphur has six valence electrons. Although sulphur has a variable valency, we know that the sulphur will be able to form 2 bonds with the hydrogen atoms. In this case, the valency of sulphur must be two.

The molecule is represented below.

Another way of representing molecules is using Couper notation . In this case, only the electrons that are involved in the bond between the atoms are shown. A line is used for each covalent bond. Using Couper notation, a molecule of water and a molecule of would be represented as shown in figures [link] and [link] below.

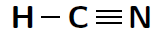

You will remember that when atoms bond, electrons are either shared or they are transferred between the atoms that are bonding. In covalent bonding, electrons are shared between the atoms. There is another type of bonding, where electrons are transferred from one atom to another. This is called ionic bonding .

Notification Switch
Would you like to follow the 'Chemistry grade 10 [caps]' conversation and receive update notifications?