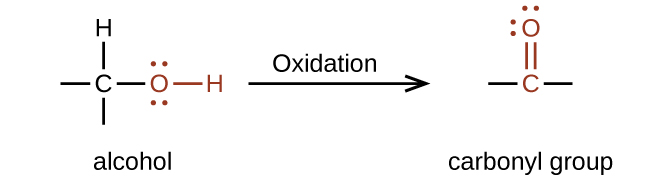
Oxidation and reduction in organic chemistry
Methane represents the completely reduced form of an organic molecule that contains one carbon atom. Sequentially replacing each of the carbon-hydrogen bonds with a carbon-oxygen bond would lead to an alcohol, then an aldehyde, then a carboxylic acid (discussed later), and, finally, carbon dioxide:
What are the oxidation numbers for the carbon atoms in the molecules shown here?
Solution
In this example, we can calculate the oxidation number (review the chapter on oxidation-reduction reactions if necessary) for the carbon atom in each case (note how this would become difficult for larger molecules with additional carbon atoms and hydrogen atoms, which is why organic chemists use the definition dealing with replacing C–H bonds with C–O bonds described). For CH
4 , the carbon atom carries a –4 oxidation number (the hydrogen atoms are assigned oxidation numbers of +1 and the carbon atom balances that by having an oxidation number of –4). For the alcohol (in this case, methanol), the carbon atom has an oxidation number of –2 (the oxygen atom is assigned –2, the four hydrogen atoms each are assigned +1, and the carbon atom balances the sum by having an oxidation number of –2; note that compared to the carbon atom in CH
4 , this carbon atom has lost two electrons so it was oxidized); for the aldehyde, the carbon atom’s oxidation number is 0 (–2 for the oxygen atom and +1 for each hydrogen atom already balances to 0, so the oxidation number for the carbon atom is 0); for the carboxylic acid, the carbon atom’s oxidation number is +2 (two oxygen atoms each at –2 and two hydrogen atoms at +1); and for carbon dioxide, the carbon atom’s oxidation number is +4 (here, the carbon atom needs to balance the –4 sum from the two oxygen atoms).
Check your learning
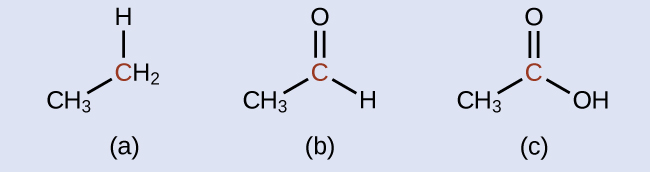
Indicate whether the marked carbon atoms in the three molecules here are oxidized or reduced relative to the marked carbon atom in ethanol:
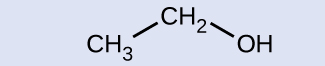
There is no need to calculate oxidation states in this case; instead, just compare the types of atoms bonded to the marked carbon atoms:
Answer:
(a) reduced (bond to oxygen atom replaced by bond to hydrogen atom); (b) oxidized (one bond to hydrogen atom replaced by one bond to oxygen atom); (c) oxidized (2 bonds to hydrogen atoms have been replaced by bonds to an oxygen atom)
Aldehydes are commonly prepared by the oxidation of alcohols whose –OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol:

Alcohols that have their –OH groups in the middle of the chain are necessary to synthesize a ketone, which requires the carbonyl group to be bonded to two other carbon atoms:

An alcohol with its –OH group bonded to a carbon atom that is bonded to no or one other carbon atom will form an aldehyde. An alcohol with its –OH group attached to two other carbon atoms will form a ketone. If three carbons are attached to the carbon bonded to the –OH, the molecule will not have a C–H bond to be replaced, so it will not be susceptible to oxidation.





