| << Chapter < Page | Chapter >> Page > |
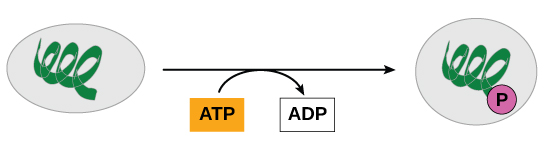
From an evolutionary perspective consider remember that the primordial ooze was hypothesized to be enriched with highly reduced small organic compounds. These reduced high energy compounds could have made an excellent initial energy source for early life. The simplest method of energy extraction being the oxidation of these compounds coupled to ATP synthesis; i.e. Substrate level phosphorylation. This simple method could theoretically have yielded large quantities of ATP for the cell along with smaller more oxidized organic compounds. Remember, we are currently only discussing ATP synthesis. If you consider this reaction, the coupling of the oxidation of reduced orgainic molecules to ATP synthesis, we are missing half the reaction, the compound to be reduced; ATP is not reduced during this reaction, it is simply forming a high energy bond. The electrons need to go somewhere, and the question where do they go?
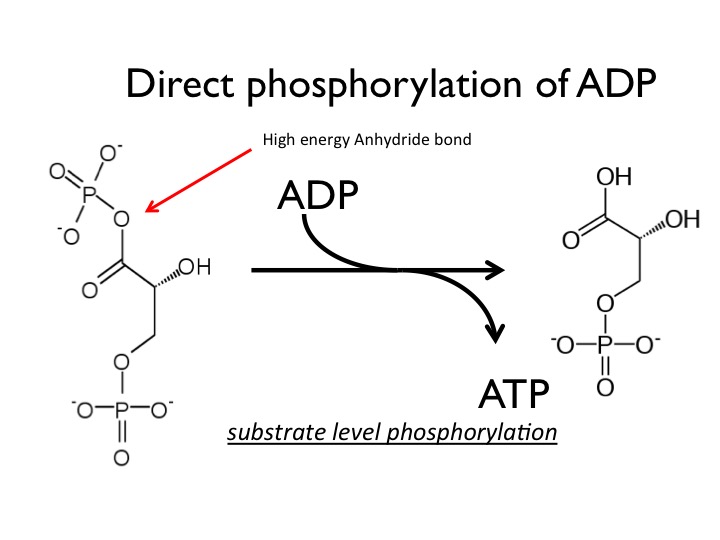
The short answer is NAD+, those co-enzymes involved in red/ox reactions. Thus the first step in the synthesis of ATP is drive a Phosphate group onto the high energy compound by a red/ox reaction. The subsequent reduction of the energy source with the simultaneous phosphorylation produces two products, NADH (from the reduction of NAD+)and the newly formed phosphorylated compound containing a high energy phosphate that can now be used to synthesize ATP in a second reaction. The best example of this in current metabolism is the reduction of glyceraldehyde-3-phosphate to 1,3-bisposphoglycerate by NAD+. The formation of 1,3-bisphosphoglycerate and a high energy phosphoanhydride can then be used to phosphorylate ADP to ATP and production of 3-phosphoglycerate. This is diagrammed in figure 5 below.

The subsequent phosphate transfer reaction to ADP to form ATP in the reaction shown in figure 6 comes directly from the formation of 1,3-bisphosphoglycerate (described above). The arrow points out the high energy phosphoanhydride that is formed in the red/ox reaction. These two enzymatic reactions demonstrate how cells can transform one form of energy into a second, the potential energy from the substrates to potential energy in ATP. And of course the energy in ATP can then be used to help drive thermodynamically unfavorable reactions in the cell.
There is one last consideration you need to think about in our discussion of SLP. Consider the question posed earlier in exercise 1. Remember NAD+ is the original oxidizing agent used to form 1,3-bisphosphoglycerate from glyceraldehyde-3-phosphate. NAD+ is a co-enzyme and its levels are finite in the cell. So, as the reactions in figures 5 and 6 occur in the cell the levels of NAD+ fall and the levels of NADH rise. If left unchecked the cell has entered a death spiral. The answer is relatively simple, regenerate NAD+ by oxidizing NADH. This process is called fermentation and its role in metabolism is to reoxidize NADH to NAD+ forming a cycle between the two forms of the same molecule; as long as there is plenty of substrate to reduce NAD+ and enough substrate to oxidize NADH back to NAD+. We will discus fermentation reactions in detail in a different module. But for this discussion, simply keep in mind that the cell must reoxidize NADH back to NAD+ in order to survive. Also keep in mind, that as with all aspects of energy metabolism, the basic process is very simple, NAD+ to NADH to NAD+ to....., yet a variety of rich complex reactions have evolved over time. This good news for us, because the end products of fermentation reactions include many foods and beverages we use every day. Think how boring life would be without bread, tea, beer and chocolate.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?