| << Chapter < Page | Chapter >> Page > |

First, as we noted before, the hydrides from Group IV are the lowest boiling point compounds in each period. Second, in each group, the boiling point increases as we move down the periodic table. In fact, this second pattern is perhaps the most pronounced trend in the data. From this, we draw our first conclusion about the strengths of intermolecular attractions: for similar types of molecules, the molecules with larger atoms (more mass, more protons) have stronger intermolecular attractions.
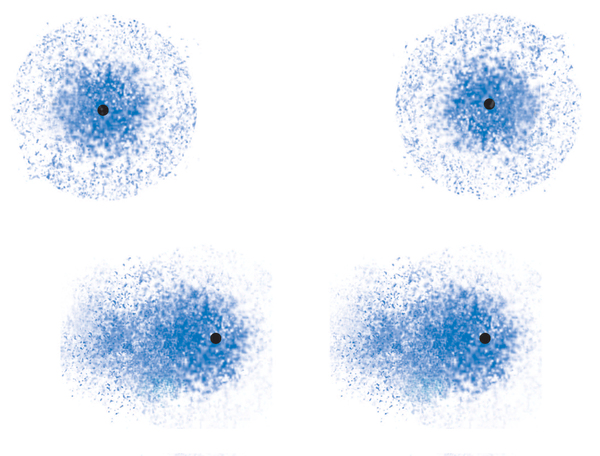
Why would this be? The answer is not obvious but does make sense once we know it. Remember that these are all neutral molecules. As such, we might have imagined that there would no positive-negative interactions. However, each molecule consists of a large of number of protons from the nuclei of the atoms, and an equal number of electrons, both core electrons and valence electrons, which are either shared or unshared. When two molecules are close to each other, the positive and negative charges in each of the molecules interact with each other. We might again imagine that the attractions of opposite charges would be exactly offset by the repulsions of like charges. This would be true if the charges were uniformly distributed in the molecules, as we would expect for non-polar molecules. But when the molecules are close enough to each other, the attractions and repulsions cause the charges to rearrange such that the attractions become significantly more favorable.
Such an arrangement of electrons in two adjacent highly simplified molecules is shown in Figure 6. Note that two nonpolar molecules become polarized when they are close to each other, due to the attraction of the negative charges in one molecule to the positive charges in the other molecule, and vice versa. The result is a net attractive force between the two molecules. This type of force is called the “dispersion force,” sometimes also called the “London force” after the discoverer.
What makes the dispersion force larger? The data in Table 1 and Figure 5 tell us: molecules with more positive and negative charges, like SnH 4 , have stronger attractions than molecules with fewer positive and negative charges, like CH 4 . We say that the molecule with more charges is more “polarizable,” meaning it is easier for the molecule to become polarized in the presence of other electrical charges. The more polarizable a molecule is, the stronger the intermolecular forces will be.
Let’s now compare the boiling points of compounds in the same period from Group IV and from Group VII, e.g. SiH 4 versus HCl. The boiling point of HCl is larger. However, if we count the charges in these two molecules, we discover that they have the same number of electrons and the same number of protons. This suggests that the two molecules should be equally polarizable and therefore should have equal dispersion forces and therefore should have equal boiling points. But this is not true. Something else must be contributing to the difference in boiling points than just the dispersion forces.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?