| << Chapter < Page | Chapter >> Page > |
Figure 4
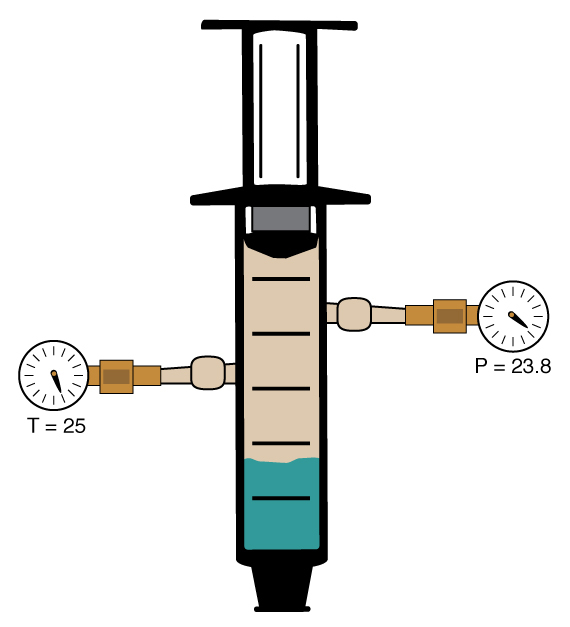
Although there was initially no gas in the container, we observe that the pressure inside the container rises to a fixed value of 23.8 torr. Clearly, there must be gaseous water in the container, since we observe a pressure inside the container. Since there was no gas initially in the container, the gas present must have come from evaporation of some of the liquid water. But not all of the liquid evaporates, since a look inside the container reveals that there is still liquid water present. Therefore, both the liquid phase and the gas phase are present at the same time in this container. This seems incongruous from our previous observations under different conditions where we found only liquid or gas to be stable.
In this case, we say that the liquid water and the gaseous water vapor are in "phase equilibrium." The term “equilibrium” in this case indicates that neither the vapor nor the liquid spontaneously converts into the other phase. In fact, at equilibrium, we don’t observe any changes in physical properties at all. The pressure of the gas remains constant, and the amount of liquid remains constant. This means that both phases are stable at equilibrium.
The concept of equilibrium occurs frequently in Chemistry in many different contexts. Phase equilibrium is one simple example where we can observe and understand equilibrium.
Let’s see what happens to the equilibrium if we change the conditions of the experiment. We can repeat our measurement by pulling the piston back to any other arbitrary position before locking it down, trapping a different volume above the liquid. We observe that the pressure in the container in every case rises to the same fixed value of 23.8 torr, provided that there is still some liquid water present. Surprisingly, it does not matter what volume we have trapped inside the cylinder, nor does it matter how much liquid water we start with. As long as there is still some liquid water present in the cylinder at equilibrium, the pressure of the vapor above that liquid is 23.8 torr at 25 ºC.
When we vary either the initial amount of liquid or the fixed volume of the container, the amount of liquid water that evaporates must be different in each case. How can we see this? The volume available for vapor to occupy is different when we either change the volume of the container or the initial volume of the liquid. Since we observe that the pressure of the vapor is the same at a fixed temperature, then the Ideal Gas Law tells us that the differing volumes correspond to differing numbers of moles of water vapor. This shows us that it is the pressure of the vapor, not the amount of vapor, which is the most important property in establishing the equilibrium between the liquid and the vapor. We can conclude that, at a fixed temperature, there is a single specific pressure at which a given liquid and its vapor will be in phase equilibrium. We call this the "vapor pressure" of the liquid.
We can make some important observations about vapor pressure. First, for a given substance, the vapor pressure varies with temperature. We can observe this by simply increasing the temperature of the closed container in the preceding experiment. In every case, we observe that the equilibrium vapor pressure always increases with increasing temperature.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?