| << Chapter < Page | Chapter >> Page > |
An example of a simple chemical reaction is the breaking down of hydrogen peroxide molecules, each of which consists of two hydrogen atoms bonded to two oxygen atoms (H 2 O 2 ). The reactant hydrogen peroxide is broken down into water, containing one oxygen atom bound to two hydrogen atoms (H 2 O), and oxygen, which consists of two bonded oxygen atoms (O 2 ). In the equation below, the reaction includes two hydrogen peroxide molecules and two water molecules. This is an example of a balanced chemical equation , wherein the number of atoms of each element is the same on each side of the equation. According to the law of conservation of matter, the number of atoms before and after a chemical reaction should be equal, such that no atoms are, under normal circumstances, created or destroyed.
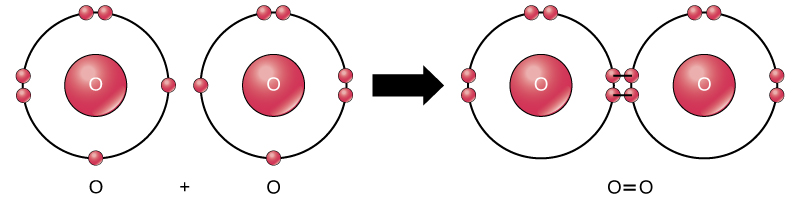
Even though all of the reactants and products of this reaction are molecules (each atom remains bonded to at least one other atom), in this reaction only hydrogen peroxide and water are representatives of compounds : they contain atoms of more than one type of element. Molecular oxygen, on the other hand, as shown in [link] ,consists of two doubly bonded oxygen atoms and is not classified as a compound but as a mononuclear molecule.
Some chemical reactions, such as the one shown above, can proceed in one direction until the reactants are all used up. The equations that describe these reactions contain a unidirectional arrow and are irreversible . Reversible reactions are those that can go in either direction. In reversible reactions, reactants are turned into products, but when the concentration of product goes beyond a certain threshold (characteristic of the particular reaction), some of these products will be converted back into reactants; at this point, the designations of products and reactants are reversed. This back and forth continues until a certain relative balance between reactants and products occurs—a state called equilibrium . These situations of reversible reactions are often denoted by a chemical equation with a double headed arrow pointing towards both the reactants and products.
For example, in human blood, excess hydrogen ions (H + ) bind to bicarbonate ions (HCO 3 - ) forming an equilibrium state with carbonic acid (H 2 CO 3 ). If carbonic acid were added to this system, some of it would be converted to bicarbonate and hydrogen ions.
In biological reactions, however, equilibrium is rarely obtained because the concentrations of the reactants or products or both are constantly changing, often with a product of one reaction being a reactant for another. To return to the example of excess hydrogen ions in the blood, the formation of carbonic acid will be the major direction of the reaction. However, the carbonic acid can also leave the body as carbon dioxide gas (via exhalation) instead of being converted back to bicarbonate ion, thus driving the reaction to the right by the chemical law known as law of mass action . These reactions are important for maintaining the homeostasis of our blood.

Notification Switch
Would you like to follow the 'Chemistry of life: bis2a modules 2.0 to 2.3 (including appendix i and ii)' conversation and receive update notifications?