| << Chapter < Page | Chapter >> Page > |
D = 230/(FWHM-50). For help on getting the FWHM use [link] .
When the nanoparticles form they are able to scatter huge amounts of light. As atoms the silver will not scatter any light, but when it is made into the form of nanoparticles the light scattering is possible to see using a simple laser light. Large chunks of silver cannot be soluble in water but it in the nanoparticle form it can be. We can use the laser pointers to see when the nanoparticles are forming.
During the first reaction you can see how sodium citrate takes a long time to create nanoparticles, at the beginning the laser does not shine through the solution. But over a period of about 20 minutes you will be able to see the nanoparticle form by using the laser light. When the nanoparticles have formed you will be able to see the laser run through the solution.
When you make the nanoparticles with sodium borohydride now, it is so strong it will make nanoparticles much faster. This reaction only takes about 2 minutes to occur.
We use light to see things around us, that light has a certain size, or wavelength. And if something is smaller than light, we cannot use the light to see it directly, so we have to use things with smaller wavelengths. Let’s use an example, consider a hand of a certain size, and some hieroglyphics on a wall( er?). With very large hands the details in the wall are difficult to make out, but still you can note that they are there. But when you use smaller and smaller hands the details become easier to make out. It’s the same kind of idea when using the light. For us as human beings it is not usually a problem in our everyday lives, the size of the light is much smaller than the artifacts we deal with as we move around. But when looking at smaller and smaller things as in the nanoscale, we can’t use visible light because the light passes right over the objects normally, and it’s as if they don’t exist.
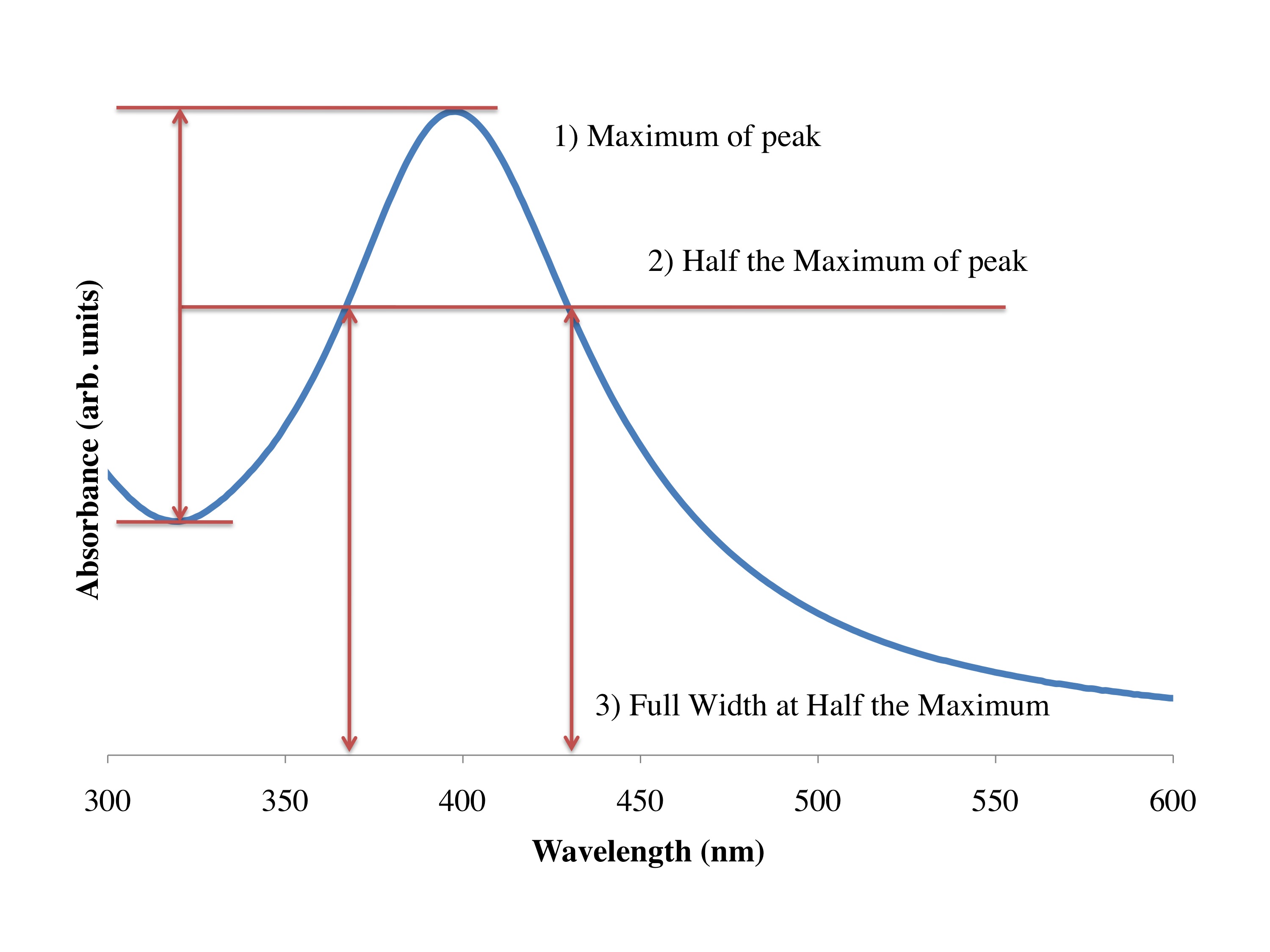
One trick around this is to use shorter wavelengths of light, like using X-rays at the hospital to image brakes and fractures of the skeletal system. And in nanotechnology what we often use are electrons, Because the wavelength of the electrons are far smaller than the object we are looking at, we can get a good picture of what is going on at the nanoscale. There are two main instruments to do this: the TEM (transmission electron microscope), and the SEM (scanning electron microscope). In the same way that the X-rays at the hospital pass through the skin but not the bones, the TEM accelerates electrons through materials, and depending on the type and size of the material the electrons either pass through or not. And we get a black and white image of our system at the nanoscale. In [link] you see a picture of the type of silver nanoparticles that you made in the lab, this was taken with a TEM in Dell Butcher Hall here at Rice. The dense silver particles don’t allow the transmission of the electrons, and we get a black and white picture of the nanoparticles. This has been calibrated and can be used to tell us the size of the particles; they are around 10 nm on average.
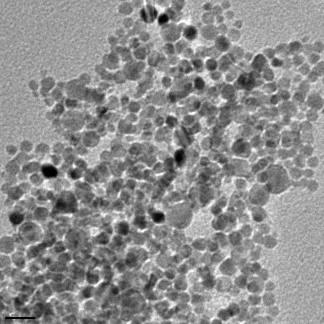
But when electrons pass through the material it is not always a clean break, some of the energy can be imparted on the materials and so it won’t pass all the way through. This can cause a secondary effect that depends on the material that is being imaged, and this is essentially how the SEM works. Instead of electrons passing through like in the X-rays in the hospital, the materials you image have a reaction to the bombardment of electrons in the electron beam. In [link] you see a bunch of larger silver nanoparticles that have been imaged using an SEM here in Dell Butcher Hall at Rice University.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?