| << Chapter < Page | Chapter >> Page > |
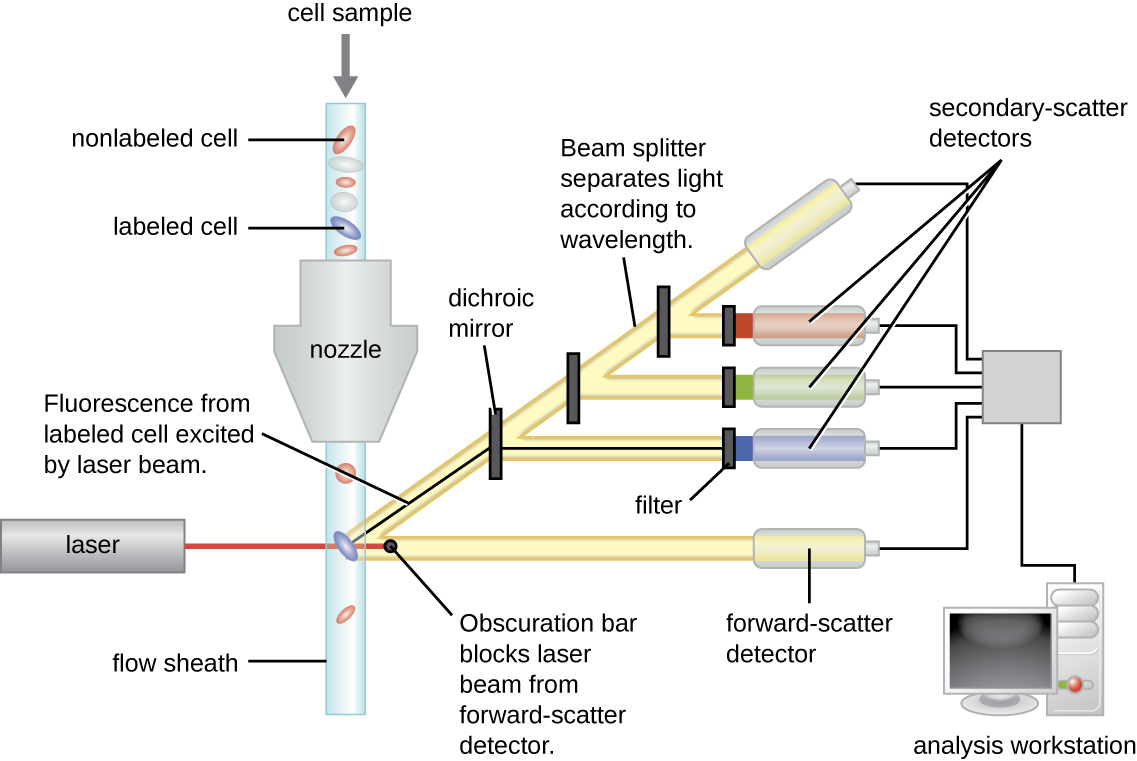
[link] shows the obscuration bar in front of the forward-scatter detector that prevents laser light from hitting the detector. As a cell passes through the laser bar, the forward-scatter detector detects light scattered around the obscuration bar. The scattered light is transformed into a voltage pulse, and the cytometer counts a cell. The fluorescence from a labeled cell is detected by the side-scatter detectors. The light passes through various dichroic mirrors such that the light emitted from the fluorophore is received by the correct detector.
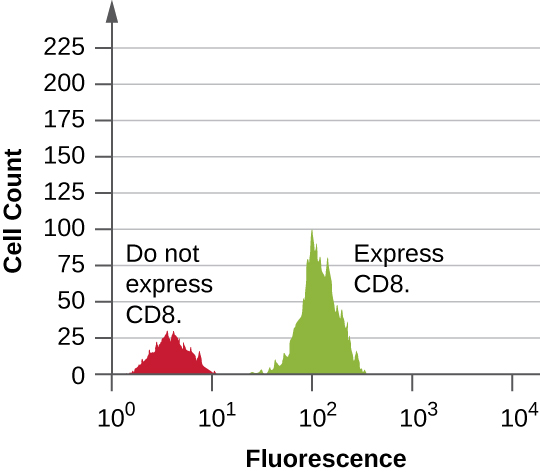
Data are collected from both the forward- and side-scatter detectors. One way these data can be presented is in the form of a histogram. The forward scatter is placed on the y -axis (to represent the number of cells), and the side scatter is placed on the x -axis (to represent the fluoresence of each cell). The scaling for the x -axis is logarithmic, so fluorescence intensity increases by a factor of 10 with each unit increase along the axis. [link] depicts an example in which a culture of cells is combined with an antibody attached to a fluorophore to detect CD8 cells and then analyzed by flow cytometry. The histogram has two peaks. The peak on the left has lower fluorescence readings, representing the subset of the cell population (approximately 30 cells) that does not fluoresce; hence, they are not bound by antibody and therefore do not express CD8. The peak on the right has higher fluorescence readings, representing the subset of the cell population (approximately 100 cells) that show fluorescence; hence, they are bound by the antibody and therefore do express CD8.
After notifying all 1300 patients, the hospital begins scheduling HIV screening. Appointments were scheduled a minimum of 3 weeks after the patient’s last hospital visit to minimize the risk of false negatives. Because some false positives were anticipated, the public health physician set up a counseling protocol for any patient whose indirect ELISA came back positive.
Of the 1300 patients, eight tested positive using the ELISA. Five of these tests were invalidated by negative western blot tests, but one western blot came back positive, confirming that the patient had indeed contracted HIV. The two remaining western blots came back indeterminate. These individuals had to submit to a third test, a PCR, to confirm the presence or absence of HIV sequences. Luckily, both patients tested negative.
As for the lone patient confirmed to have HIV, the tests cannot prove or disprove any connection to the syringes compromised by the former hospital employee. Even so, the hospital’s insurance will fully cover the patient’s treatment, which began immediately.
Although we now have drugs that are typically effective at controlling the progression of HIV and AIDS, there is still no cure. If left untreated, or if the drug regimen fails, the patient will experience a gradual decline in the number of CD4 helper T cells, resulting in severe impairment of all adaptive immune functions. Even moderate declines of helper T cell numbers can result in immunodeficiency, leaving the patient susceptible to opportunistic infections. To monitor the status of the patient’s helper T cells, the hospital will use flow cytometry. This sensitive test allows physicians to precisely determine the number of helper T cells so they can adjust treatment if the number falls below 500 cells/µL.
Jump to the previous Clinical Focus box.
The flow cytometer and immunofluorescence can also be modified to sort cells from a single sample into purified subpopulations of cells for research purposes. This modification of the flow cytometer is called a fluorescence-activated cell sorter (FACS) . In a FACS, fluorescence by a cell induces the device to put a charge on a droplet of the transporting fluid containing that cell. The charge is specific to the wavelength of the fluorescent light, which allows for differential sorting by those different charges. The sorting is accomplished by an electrostatic deflector that moves the charged droplet containing the cell into one collecting vessel or another. The process results in highly purified subpopulations of cells.
One limitation of a FACS is that it only works on isolated cells. Thus, the method would work in sorting white blood cells, since they exist as isolated cells. But for cells in a tissue, flow cytometry can only be applied if we can excise the tissue and separate it into single cells (using proteases to cleave cell-cell adhesion molecules) without disrupting cell integrity. This method may be used on tumors, but more often, immunohistochemistry and immunocytochemistry are used to study cells in tissues.

Watch videos to learn more about how flow cytometry and a FACS work.
[link] compares the mechanisms of the fluorescent antibody techniques discussed in this section.
| Fluorescent Antibody Techniques | ||
|---|---|---|
| Type of Assay | Mechanism | Examples |
| Direct fluorescent antibody (DFA) | Uses fluorogen-antibody conjugates to label bacteria from patient samples | Visualizing Legionella pneumophila from a throat swab |
| Indirect fluorescent antibody (IFA) | Detects disease-specific antibodies in patent serum | Diagnosing syphilis; detecting antinuclear antibodies (ANA) for lupus and other autoimmune diseases |
| Flow cytometry | Labels cell membranes with fluorogen-antibody conjugate markers excited by a laser; machine counts the cell and records the relative fluorescence | Counting the number of fluorescently labeled CD4 or CD8 cells in a sample |
| Fluorescence activated cell sorter (FACS) | Form of flow cytometry that both counts cells and physically separates them into pools of high and low fluorescence cells | Sorting cancer cells |
In flow cytometry, cell subsets are labeled using a fluorescent antibody to a membrane protein. The fluorogen is activated by a(n) ________ as the cells pass by the detectors.
laser
Fluorescence in a flow cytometer is measured by a detector set at an angle to the light source. There is also an in-line detector that can detect cell clumps or ________.
fragments

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?