| << Chapter < Page | Chapter >> Page > |
Fermentation by some bacteria, like those in yogurt and other soured food products, and by animals in muscles during oxygen depletion, is lactic acid fermentation . The chemical reaction of lactic acid fermentation is as follows:
Bacteria of several gram-positive genera, including Lactobacillus , Leuconostoc , and Streptococcus , are collectively known as the lactic acid bacteria (LAB) , and various strains are important in food production. During yogurt and cheese production, the highly acidic environment generated by lactic acid fermentation denatures proteins contained in milk, causing it to solidify. When lactic acid is the only fermentation product, the process is said to be homolactic fermentation ; such is the case for Lactobacillus delbrueckii and S. thermophiles used in yogurt production. However, many bacteria perform heterolactic fermentation , producing a mixture of lactic acid, ethanol and/or acetic acid, and CO 2 as a result, because of their use of the branched pentose phosphate pathway instead of the EMP pathway for glycolysis. One important heterolactic fermenter is Leuconostoc mesenteroides , which is used for souring vegetables like cucumbers and cabbage, producing pickles and sauerkraut, respectively.
Lactic acid bacteria are also important medically. The production of low pH environments within the body inhibits the establishment and growth of pathogens in these areas. For example, the vaginal microbiota is composed largely of lactic acid bacteria, but when these bacteria are reduced, yeast can proliferate, causing a yeast infection. Additionally, lactic acid bacteria are important in maintaining the health of the gastrointestinal tract and, as such, are the primary component of probiotics.
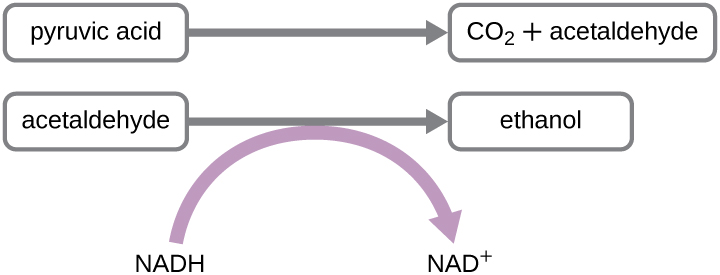
Another familiar fermentation process is alcohol fermentation , which produces ethanol. The ethanol fermentation reaction is shown in [link] . In the first reaction, the enzyme pyruvate decarboxylase removes a carboxyl group from pyruvate, releasing CO 2 gas while producing the two-carbon molecule acetaldehyde. The second reaction, catalyzed by the enzyme alcohol dehydrogenase, transfers an electron from NADH to acetaldehyde, producing ethanol and NAD + . The ethanol fermentation of pyruvate by the yeast Saccharomyces cerevisiae is used in the production of alcoholic beverages and also makes bread products rise due to CO 2 production. Outside of the food industry, ethanol fermentation of plant products is important in biofuel production.
Beyond lactic acid fermentation and alcohol fermentation, many other fermentation methods occur in prokaryotes, all for the purpose of ensuring an adequate supply of NAD + for glycolysis ( [link] ). Without these pathways, glycolysis would not occur and no ATP would be harvested from the breakdown of glucose. It should be noted that most forms of fermentation besides homolactic fermentation produce gas, commonly CO 2 and/or hydrogen gas. Many of these different types of fermentation pathways are also used in food production and each results in the production of different organic acids, contributing to the unique flavor of a particular fermented food product. The propionic acid produced during propionic acid fermentation contributes to the distinctive flavor of Swiss cheese, for example.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?