| << Chapter < Page | Chapter >> Page > |
The size (length) and specific amino acid sequence of a protein are major determinants of its shape, and the shape of a protein is critical to its function. For example, in the process of biological nitrogen fixation (see Biogeochemical Cycles ), soil microorganisms collectively known as rhizobia symbiotically interact with roots of legume plants such as soybeans, peanuts, or beans to form a novel structure called a nodule on the plant roots. The plant then produces a carrier protein called leghemoglobin, a protein that carries nitrogen or oxygen. Leghemoglobin binds with a very high affinity to its substrate oxygen at a specific region of the protein where the shape and amino acid sequence are appropriate (the active site ). If the shape or chemical environment of the active site is altered, even slightly, the substrate may not be able to bind as strongly, or it may not bind at all. Thus, for the protein to be fully active, it must have the appropriate shape for its function.
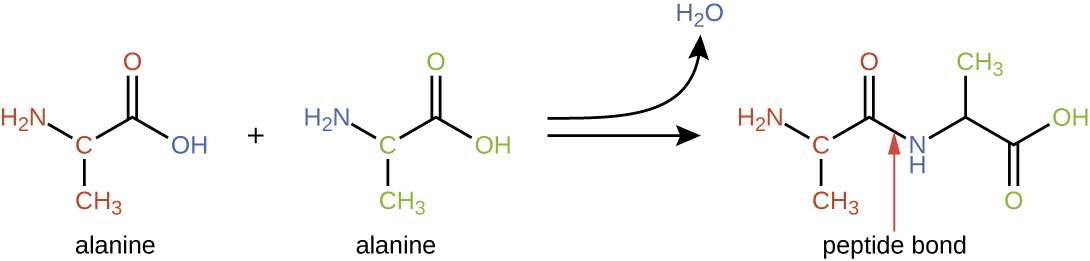
Protein structure is categorized in terms of four levels: primary, secondary, tertiary, and quaternary. The primary structure is simply the sequence of amino acid s that make up the polypeptide chain . [link] depicts the primary structure of a protein.
The chain of amino acids that defines a protein’s primary structure is not rigid, but instead is flexible because of the nature of the bonds that hold the amino acids together. When the chain is sufficiently long, hydrogen bonding may occur between amine and carbonyl functional groups within the peptide backbone (excluding the R side group), resulting in localized folding of the polypeptide chain into helices and sheets. These shapes constitute a protein’s secondary structure . The most common secondary structures are the α-helix and β-pleated sheet. In the α-helix structure, the helix is held by hydrogen bonds between the oxygen atom in a carbonyl group of one amino acid and the hydrogen atom of the amino group that is just four amino acid units farther along the chain. In the β-pleated sheet , the pleats are formed by similar hydrogen bonds between continuous sequences of carbonyl and amino groups that are further separated on the backbone of the polypeptide chain ( [link] ).
The next level of protein organization is the tertiary structure , which is the large-scale three-dimensional shape of a single polypeptide chain. Tertiary structure is determined by interactions between amino acid residues that are far apart in the chain. A variety of interactions give rise to protein tertiary structure, such as disulfide bridge s, which are bonds between the sulfhydryl (–SH) functional groups on amino acid side groups; hydrogen bonds; ionic bonds; and hydrophobic interactions between nonpolar side chains. All these interactions, weak and strong, combine to determine the final three-dimensional shape of the protein and its function ( [link] ).

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?