| << Chapter < Page | Chapter >> Page > |
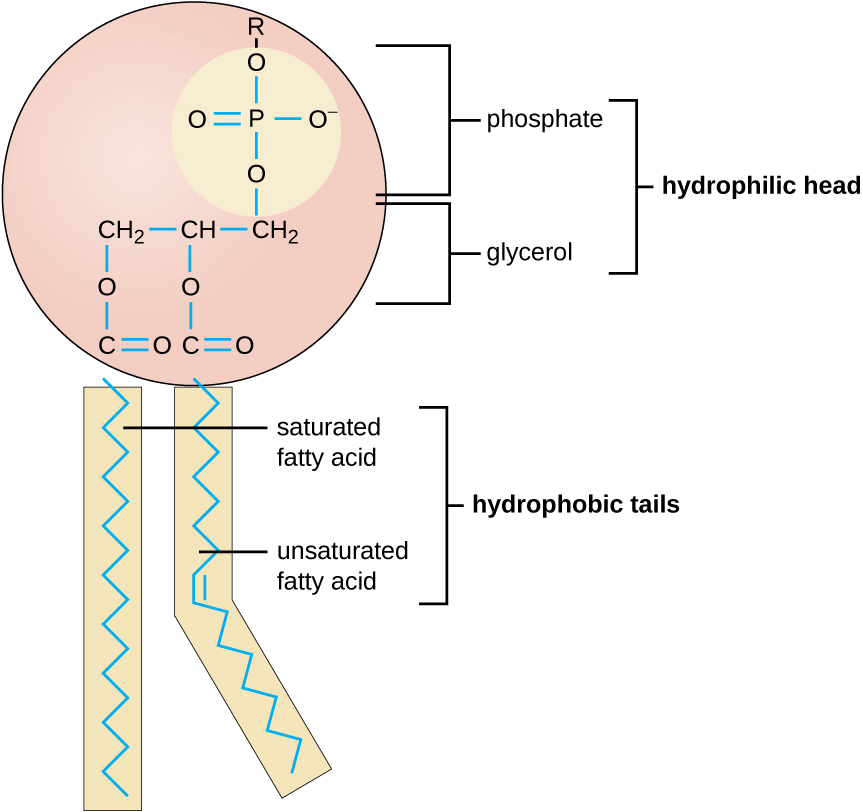
The molecular structure of lipids results in unique behavior in aqueous environments. [link] depicts the structure of a triglyceride . Because all three substituents on the glycerol backbone are long hydrocarbon chains, these compounds are nonpolar and not significantly attracted to polar water molecules—they are hydrophobic. Conversely, phospholipid s such as the one shown in [link] have a negatively charged phosphate group. Because the phosphate is charged, it is capable of strong attraction to water molecules and thus is hydrophilic , or “water loving.” The hydrophilic portion of the phospholipid is often referred to as a polar “head,” and the long hydrocarbon chains as nonpolar “tails.” A molecule presenting a hydrophobic portion and a hydrophilic moiety is said to be amphipathic . Notice the “R” designation within the hydrophilic head depicted in [link] , indicating that a polar head group can be more complex than a simple phosphate moiety. Glycolipids are examples in which carbohydrates are bonded to the lipids’ head groups.
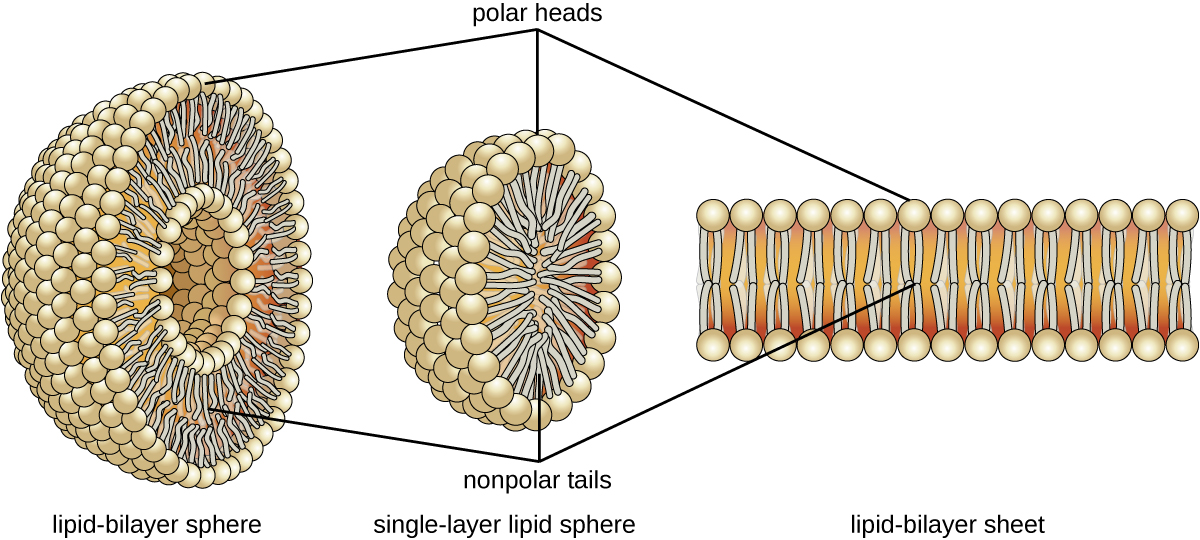
The amphipathic nature of phospholipids enables them to form uniquely functional structures in aqueous environments. As mentioned, the polar heads of these molecules are strongly attracted to water molecules, and the nonpolar tails are not. Because of their considerable lengths, these tails are, in fact, strongly attracted to one another. As a result, energetically stable, large-scale assemblies of phospholipid molecules are formed in which the hydrophobic tails congregate within enclosed regions, shielded from contact with water by the polar heads ( [link] ). The simplest of these structures are micelle s , spherical assemblies containing a hydrophobic interior of phospholipid tails and an outer surface of polar head groups. Larger and more complex structures are created from lipid-bilayer sheets, or unit membranes , which are large, two-dimensional assemblies of phospholipid s congregated tail to tail. The cell membranes of nearly all organisms are made from lipid-bilayer sheets, as are the membranes of many intracellular components. These sheets may also form lipid-bilayer spheres that are the structural basis of vesicle s and liposome s, subcellular components that play a role in numerous physiological functions.
The isoprenoids are branched lipids, also referred to as terpenoids , that are formed by chemical modifications of the isoprene molecule ( [link] ). These lipids play a wide variety of physiological roles in plants and animals, with many technological uses as pharmaceuticals (capsaicin), pigments (e.g., orange beta carotene, xanthophylls), and fragrances (e.g., menthol, camphor, limonene [lemon fragrance], and pinene [pine fragrance]). Long-chain isoprenoids are also found in hydrophobic oils and waxes . Waxes are typically water resistant and hard at room temperature, but they soften when heated and liquefy if warmed adequately. In humans, the main wax production occurs within the sebaceous glands of hair follicles in the skin, resulting in a secreted material called sebum, which consists mainly of triacylglycerol, wax esters, and the hydrocarbon squalene. There are many bacteria in the microbiota on the skin that feed on these lipids. One of the most prominent bacteria that feed on lipids is Propionibacterium acnes , which uses the skin’s lipids to generate short-chain fatty acids and is involved in the production of acne.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?