| << Chapter < Page | Chapter >> Page > |
Carbon chains form the skeletons of most organic molecules. Functional groups combine with the chain to form biomolecules. Because these biomolecules are typically large, we call them macromolecule s. Many biologically relevant macromolecules are formed by linking together a great number of identical, or very similar, smaller organic molecules. The smaller molecules act as building blocks and are called monomer s , and the macromolecules that result from their linkage are called polymer s . Cells and cell structures include four main groups of carbon-containing macromolecules: polysaccharides , proteins , lipids , and nucleic acids . The first three groups of molecules will be studied throughout this chapter. The biochemistry of nucleic acids will be discussed in Biochemistry of the Genome .
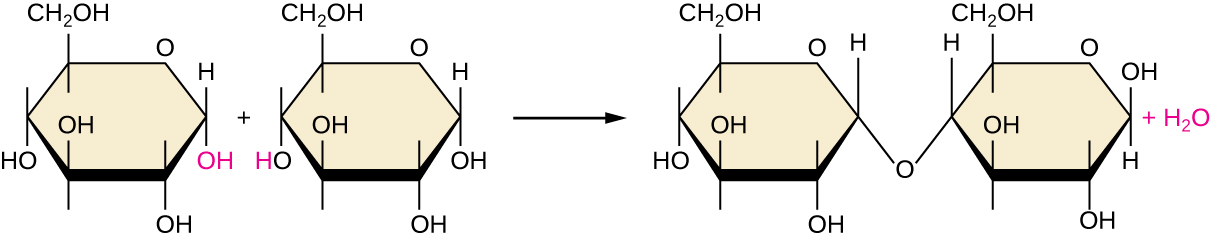
Of the many possible ways that monomers may be combined to yield polymers, one common approach encountered in the formation of biological macromolecules is dehydration synthesis . In this chemical reaction, monomer molecules bind end to end in a process that results in the formation of water molecules as a byproduct:
[link] shows dehydration synthesis of glucose binding together to form maltose and a water molecule. [link] summarizes macromolecules and some of their functions.
| Some Functions of Macromolecules | |
|---|---|
| Macromolecule | Functions |
| Carbohydrates | Energy storage, receptors, food, structural role in plants, fungal cell walls, exoskeletons of insects |
| Lipids | Energy storage, membrane structure, insulation, hormones, pigments |
| Nucleic acids | Storage and transfer of genetic information |
| Proteins | Enzymes, structure, receptors, transport, structural role in the cytoskeleton of a cell and the extracellular matrix |
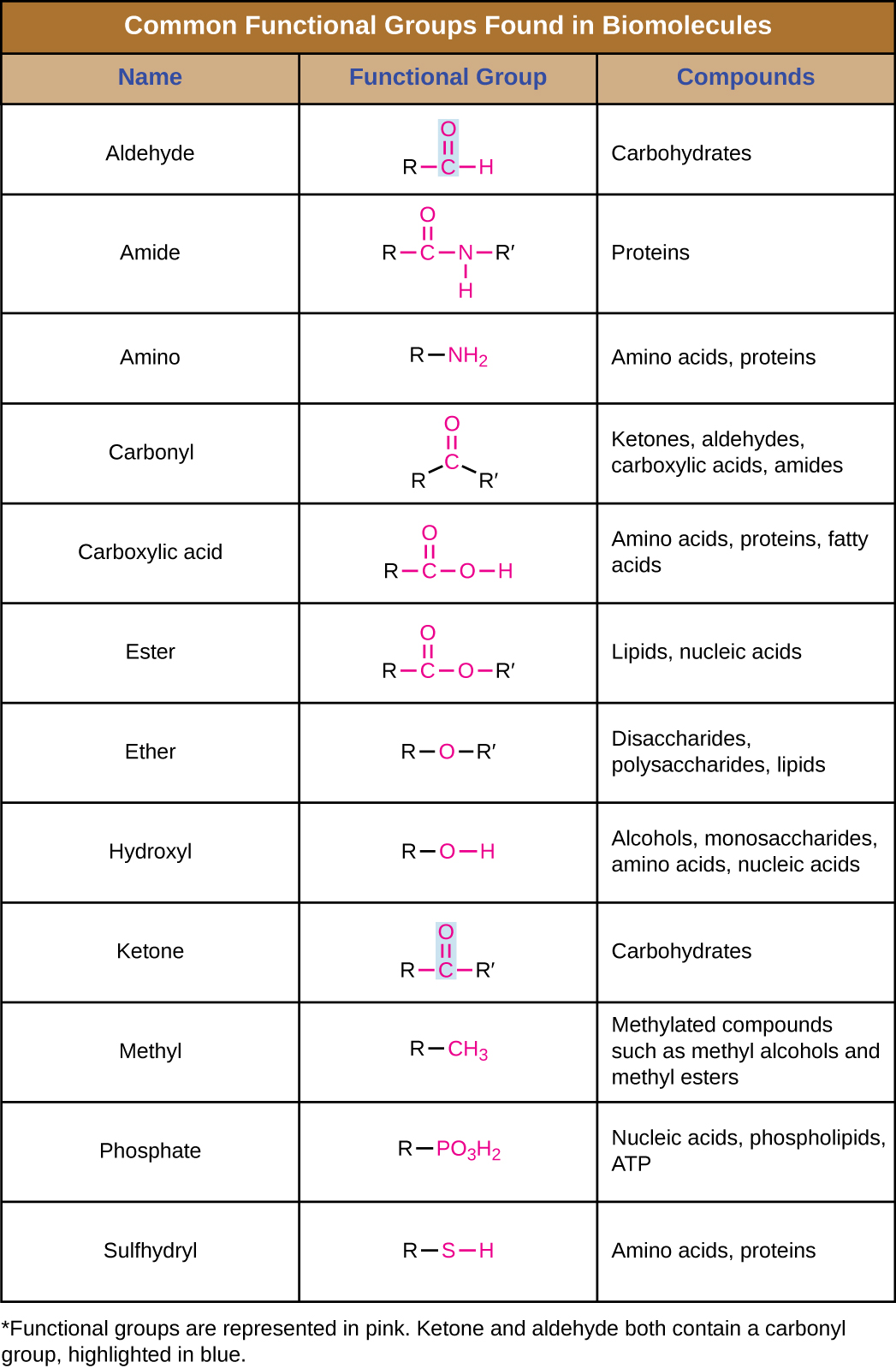
Aldehydes, amides, carboxylic acids, esters, and ketones all contain carbonyl groups.
True
Two molecules containing the same types and numbers of atoms but different bonding sequences are called enantiomers.
False
Why are carbon, nitrogen, oxygen, and hydrogen the most abundant elements in living matter and, therefore, considered macronutrients?
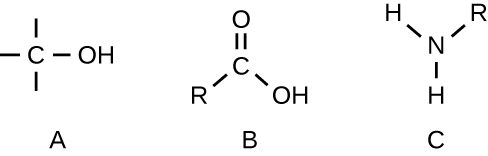
Identify the functional group in each of the depicted structural formulas.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?