| << Chapter < Page | Chapter >> Page > |
Penny is a 16-year-old student who visited her doctor, complaining about an itchy skin rash. She had a history of allergic episodes. The doctor looked at her sun-tanned skin and asked her if she switched to a different sunscreen. She said she had, so the doctor diagnosed an allergic eczema. The symptoms were mild so the doctor told Penny to avoid using the sunscreen that caused the reaction and prescribed an over-the-counter moisturizing cream to keep her skin hydrated and to help with itching.
Jump to the next Clinical Focus box.
Biochemistry is the discipline that studies the chemistry of life, and its objective is to explain form and function based on chemical principles. Organic chemistry is the discipline devoted to the study of carbon-based chemistry, which is the foundation for the study of biomolecules and the discipline of biochemistry . Both biochemistry and organic chemistry are based on the concepts of general chemistry, some of which are presented in Appendix A .
The most abundant element in cells is hydrogen (H), followed by carbon (C), oxygen (O), nitrogen (N), phosphorous (P), and sulfur (S). We call these elements macronutrient s , and they account for about 99% of the dry weight of cells. Some elements, such as sodium (Na), potassium (K), magnesium (Mg), zinc (Zn), iron (Fe), calcium (Ca), molybdenum (Mo), copper (Cu), cobalt (Co), manganese (Mn), or vanadium (Va), are required by some cells in very small amounts and are called micronutrient s or trace element s . All of these elements are essential to the function of many biochemical reactions, and, therefore, are essential to life.
The four most abundant elements in living matter (C, N, O, and H) have low atomic numbers and are thus light elements capable of forming strong bonds with other atoms to produce molecules ( [link] ). Carbon forms four chemical bonds, whereas nitrogen forms three, oxygen forms two, and hydrogen forms one. When bonded together within molecules, oxygen, sulfur, and nitrogen often have one or more “lone pairs” of electrons that play important roles in determining many of the molecules’ physical and chemical properties (see Appendix A ). These traits in combination permit the formation of a vast number of diverse molecular species necessary to form the structures and enable the functions of living organisms.
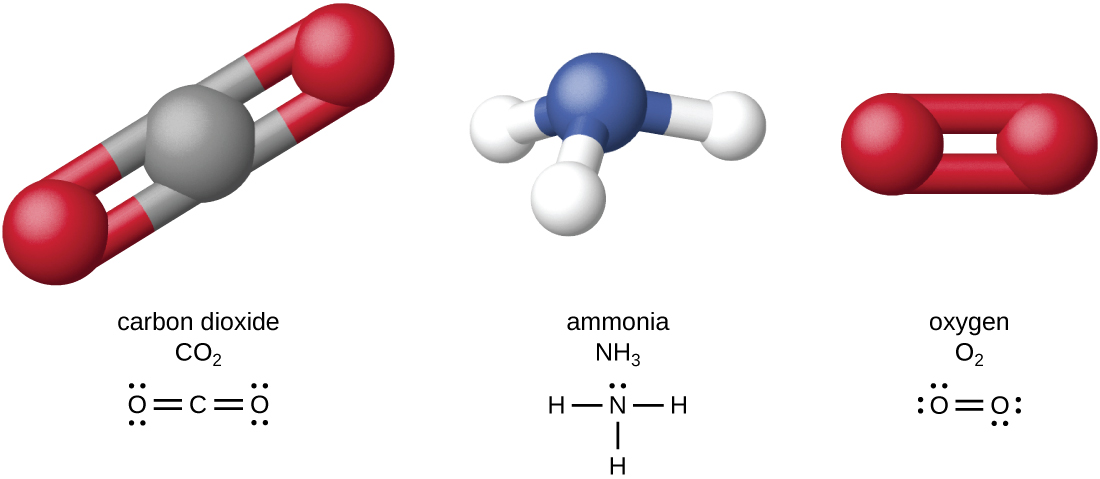
Living organisms contain inorganic compound s (mainly water and salts; see Appendix A ) and organic molecules. Organic molecules contain carbon; inorganic compounds do not. Carbon oxides and carbonates are exceptions; they contain carbon but are considered inorganic because they do not contain hydrogen. The atoms of an organic molecule are typically organized around chains of carbon atoms.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?