| << Chapter < Page | Chapter >> Page > |
Labor force : the number of employed plus the unemployed
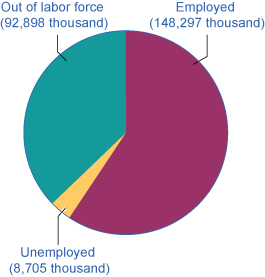
[link] shows the three-way division of the over-16 adult population. In February 2015, about 62.8% of the adult population was "in the labor force"; that is, people are either employed or without a job but looking for work. Those in the labor force can be divided into the employed and the unemployed. These values are also shown in [link] . The unemployment rate is not the percentage of the total adult population without jobs, but rather the percentage of adults who are in the labor force but who do not have jobs:
| Total adult population over the age of 16 | 249.9 million |
| In the labor force | 157 million (62.8%) |
| Employed | 148.3 million |
| Unemployed | 8.7 million |
| Out of the labor force | 92.9 million (37.2%) |
In this example, the unemployment rate can be calculated as 8.7 million unemployed people divided by 157 million people in the labor force, which works out to a 5.5% rate of unemployment. The following Work It Out feature will walk you through the steps of this calculation.
So how do economists arrive at the percentages in and out of the labor force and the unemployment rate? We will use the values in [link] to illustrate the steps.
To determine the percentage in the labor force:
Step 1. Divide the number of people in the labor force (157 million) by the total adult (working-age) population (249.9 million).
Step 2. Multiply by 100 to obtain the percentage.
To determine the percentage out of the labor force:
Step 1. Divide the number of people out the labor force (92.9 million) by the total adult (working-age) population (249.9 million).
Step 2. Multiply by 100 to obtain the percentage.
To determine the unemployment rate:
Step 1. Divide the number of unemployed people (8.7 million) by the total labor force (157 million).
Step 2. Multiply by 100 to obtain the rate.
Even with the “out of the labor force” category, there are still some people that are mislabeled in the categorization of employed, unemployed, or out of the labor force. There are some people who have only part time or temporary jobs and who are looking for full time and permanent employment that are counted as employed, though they are not employed in the way they would like or need to be. Additionally, there are individuals who are underemployed . This includes those that are trained or skilled for one type or level of work who are working in a lower paying job or one that does not utilize their skills. For example, an individual with a college degree in finance who is working as a sales clerk would be considered underemployed. They are, however, also counted in the employed group. All of these individuals fall under the umbrella of the term “ hidden unemployment .” Discouraged workers , those who have stopped looking for employment and, hence, are no longer counted in the unemployed also fall into this group

Notification Switch
Would you like to follow the 'Macroeconomics' conversation and receive update notifications?