| << Chapter < Page | Chapter >> Page > |
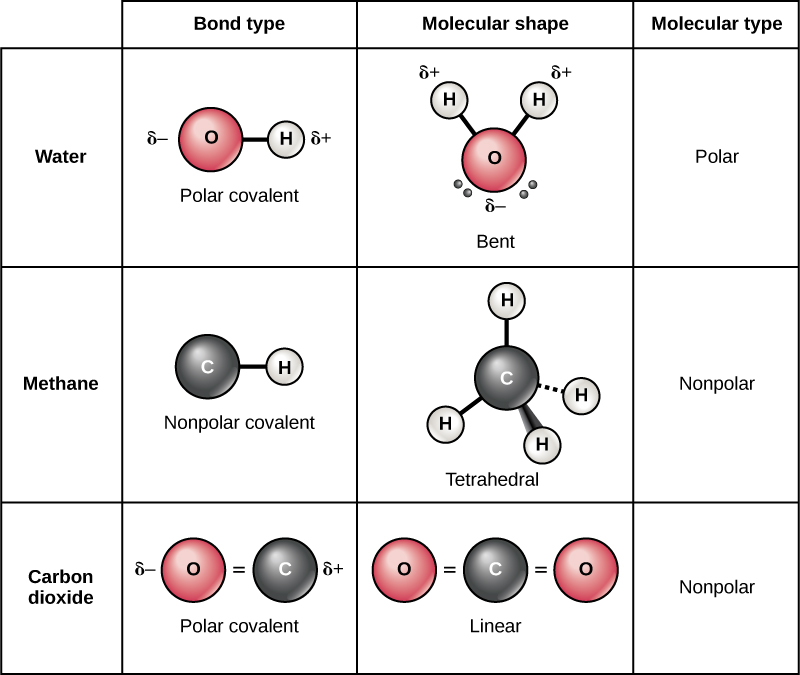
There are two types of covalent bonds: polar and nonpolar. In a polar covalent bond , shown in [link] , the electrons are unequally shared by the atoms and are attracted more to one nucleus than the other. Because of the unequal distribution of electrons between the atoms of different elements, a slightly positive ( δ +) or slightly negative ( δ –) charge develops. This partial charge is an important property of water and accounts for many of its characteristics.
Water is a polar molecule, with the hydrogen atoms acquiring a partial positive charge and the oxygen a partial negative charge. This occurs because the nucleus of the oxygen atom is more attractive to the electrons of the hydrogen atoms than the hydrogen nucleus is to the oxygen’s electrons. Thus oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms, giving the atoms of oxygen and hydrogen slightly negative and positive charges, respectively. Another way of stating this is that the probability of finding a shared electron near an oxygen nucleus is more likely than finding it near a hydrogen nucleus. Either way, the atom’s relative electronegativity contributes to the development of partial charges whenever one element is significantly more electronegative than the other, and the charges generated by these polar bonds may then be used for the formation of hydrogen bonds based on the attraction of opposite partial charges. (Hydrogen bonds, which are discussed in detail below, are weak bonds between slightly positively charged hydrogen atoms to slightly negatively charged atoms in other molecules.) Since macromolecules often have atoms within them that differ in electronegativity, polar bonds are often present in organic molecules.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O 2 ) is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
Another example of a nonpolar covalent bond is methane (CH 4 ), also shown in [link] . Carbon has four electrons in its outermost shell and needs four more to fill it. It gets these four from four hydrogen atoms, each atom providing one, making a stable outer shell of eight electrons. Carbon and hydrogen do not have the same electronegativity but are similar; thus, nonpolar bonds form. The hydrogen atoms each need one electron for their outermost shell, which is filled when it contains two electrons. These elements share the electrons equally among the carbons and the hydrogen atoms, creating a nonpolar covalent molecule.
Ionic and covalent bonds between elements require energy to break. Iconic bonds are not as strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can also form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells.
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs between the δ + of the hydrogen from one molecule and the δ – charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the same molecule. This interaction is called a hydrogen bond . This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which are not always symmetrical around an atom. For these attractions to happen, the molecules need to be very close to one another. These bonds, along with hydrogen bonds, help form the three-dimensional structure of the proteins in our cells that is necessary for their proper function.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?