| << Chapter < Page | Chapter >> Page > |
Many enzymes do not work optimally, or even at all, unless bound to other specific non-protein helper molecules. They may bond either temporarily through ionic or hydrogen bonds, or permanently through stronger covalent bonds. Binding to these molecules promotes optimal shape and function of their respective enzymes. Two examples of these types of helper molecules are cofactors and coenzymes. Cofactors are inorganic ions such as ions of iron and magnesium. Coenzymes are organic helper molecules, those with a basic atomic structure made up of carbon and hydrogen. Like enzymes, these molecules participate in reactions without being changed themselves and are ultimately recycled and reused. Vitamins are the source of coenzymes. Some vitamins are the precursors of coenzymes and others act directly as coenzymes. Vitamin C is a direct coenzyme for multiple enzymes that take part in building the important connective tissue, collagen. Therefore, enzyme function is, in part, regulated by the abundance of various cofactors and coenzymes, which may be supplied by an organism’s diet or, in some cases, produced by the organism.
Molecules can regulate enzyme function in many ways. The major question remains, however: What are these molecules and where do they come from? Some are cofactors and coenzymes, as you have learned. What other molecules in the cell provide enzymatic regulation such as allosteric modulation, and competitive and non-competitive inhibition? Perhaps the most relevant sources of regulatory molecules, with respect to enzymatic cellular metabolism, are the products of the cellular metabolic reactions themselves. In a most efficient and elegant way, cells have evolved to use the products of their own reactions for feedback inhibition of enzyme activity. Feedback inhibition involves the use of a reaction product to regulate its own further production ( [link] ). The cell responds to an abundance of the products by slowing down production during anabolic or catabolic reactions. Such reaction products may inhibit the enzymes that catalyzed their production through the mechanisms described above.
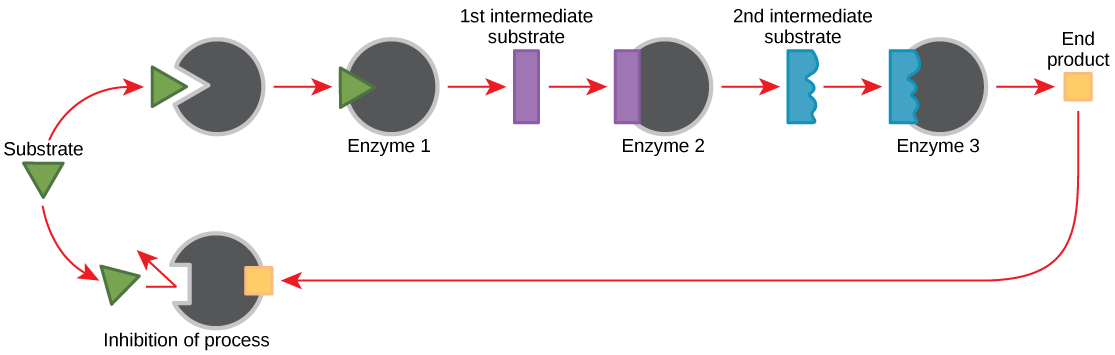
The production of both amino acids and nucleotides is controlled through feedback inhibition. Additionally, ATP is an allosteric regulator of some of the enzymes involved in the catabolic breakdown of sugar, the process that creates ATP. In this way, when ATP is in abundant supply, the cell can prevent the production of ATP. On the other hand, ADP serves as a positive allosteric regulator (an allosteric activator) for some of the same enzymes that are inhibited by ATP. Thus, when relative levels of ADP are high compared to ATP, the cell is triggered to produce more ATP through sugar catabolism.
Cells perform the functions of life through various chemical reactions. A cell’s metabolism refers to the combination of chemical reactions that take place within it. Catabolic reactions break down complex chemicals into simpler ones and are associated with energy release. Anabolic processes build complex molecules out of simpler ones and require energy.
In studying energy, the term system refers to the matter and environment involved in energy transfers. Entropy is a measure of the disorder of a system. The physical laws that describe the transfer of energy are the laws of thermodynamics. The first law states that the total amount of energy in the universe is constant. The second law of thermodynamics states that every energy transfer involves some loss of energy in an unusable form, such as heat energy. Energy comes in different forms: kinetic, potential, and free. The change in free energy of a reaction can be negative (releases energy, exergonic) or positive (consumes energy, endergonic). All reactions require an initial input of energy to proceed, called the activation energy.
Enzymes are chemical catalysts that speed up chemical reactions by lowering their activation energy. Enzymes have an active site with a unique chemical environment that fits particular chemical reactants for that enzyme, called substrates. Enzymes and substrates are thought to bind according to an induced-fit model. Enzyme action is regulated to conserve resources and respond optimally to the environment.
[link] Look at each of the processes shown and decide if it is endergonic or exergonic.
[link] A compost pile decomposing is an exergonic process. A baby developing from a fertilized egg is an endergonic process. Tea dissolving into water is an exergonic process. A ball rolling downhill is an exergonic process.

Notification Switch
Would you like to follow the 'Concepts of biology' conversation and receive update notifications?