| << Chapter < Page | Chapter >> Page > |
The motion of muscle shortening occurs as myosin heads bind to actin and pull the actin inwards. This action requires energy, which is provided by ATP. Myosin binds to actin at a binding site on the actin protein. Myosin has another binding site for ATP at which enzymatic activity hydrolyzes ATP to ADP, releasing an inorganic phosphate molecule and energy.
ATP binding causes myosin to release actin, allowing actin and myosin to detach from each other. After this happens, the newly bound ATP is converted to ADP and inorganic phosphate, P i . The enzyme at the binding site on myosin is called ATPase. The energy released during ATP hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin head is then in a position for further movement, possessing potential energy, but ADP and P i are still attached. If actin binding sites are covered and unavailable, the myosin will remain in the high energy configuration with ATP hydrolyzed, but still attached.
If the actin binding sites are uncovered, a cross-bridge will form; that is, the myosin head spans the distance between the actin and myosin molecules. P i is then released, allowing myosin to expend the stored energy as a conformational change. The myosin head moves toward the M line, pulling the actin along with it. As the actin is pulled, the filaments move approximately 10 nm toward the M line. This movement is called the power stroke, as it is the step at which force is produced. As the actin is pulled toward the M line, the sarcomere shortens and the muscle contracts.
When the myosin head is “cocked,” it contains energy and is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke; at the end of the power stroke, the myosin head is in a low-energy position. After the power stroke, ADP is released; however, the cross-bridge formed is still in place, and actin and myosin are bound together. ATP can then attach to myosin, which allows the cross-bridge cycle to start again and further muscle contraction can occur ( [link] ).
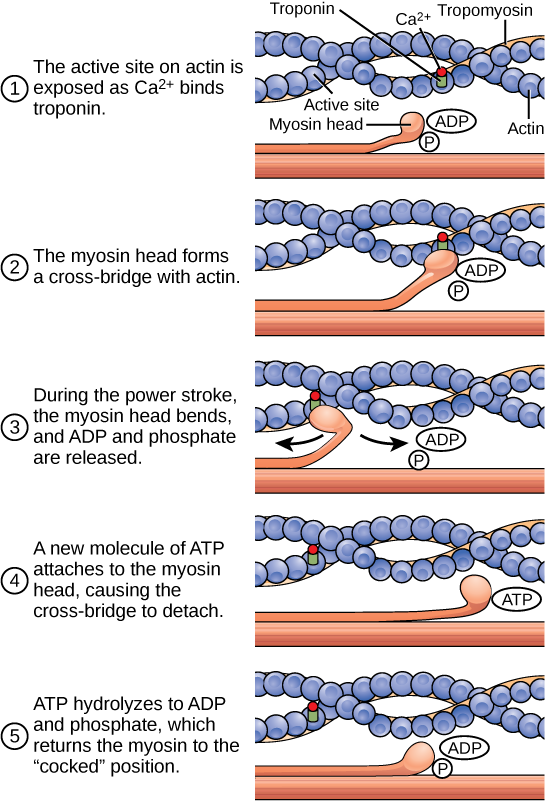
When a muscle is in a resting state, actin and myosin are separated. To keep actin from binding to the active site on myosin, regulatory proteins block the molecular binding sites. Tropomyosin blocks myosin binding sites on actin molecules, preventing cross-bridge formation and preventing contraction in a muscle without nervous input. Troponin binds to tropomyosin and helps to position it on the actin molecule; it also binds calcium ions.
To enable a muscle contraction, tropomyosin must change conformation, uncovering the myosin-binding site on an actin molecule and allowing cross-bridge formation. This can only happen in the presence of calcium, which is kept at extremely low concentrations in the sarcoplasm. If present, calcium ions bind to troponin, causing conformational changes in troponin that allow tropomyosin to move away from the myosin binding sites on actin. Once the tropomyosin is removed, a cross-bridge can form between actin and myosin, triggering contraction. Cross-bridge cycling continues until Ca 2+ ions and ATP are no longer available and tropomyosin again covers the binding sites on actin.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?