| << Chapter < Page | Chapter >> Page > |

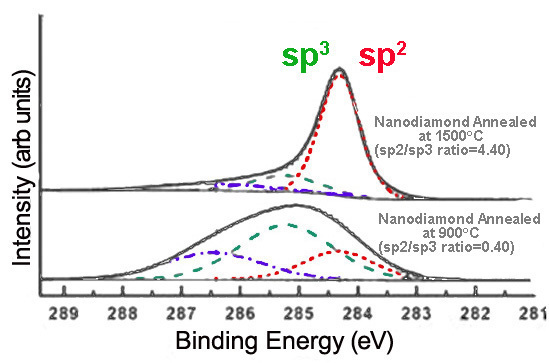
Alternatively, annealing nanodiamond thin films at very high temperatures creates graphitic layers on the nanodiamond surface, increasing sp 2 content. The extent of graphitization increases with the temperature at which the sample is annealed, as shown in [link] .
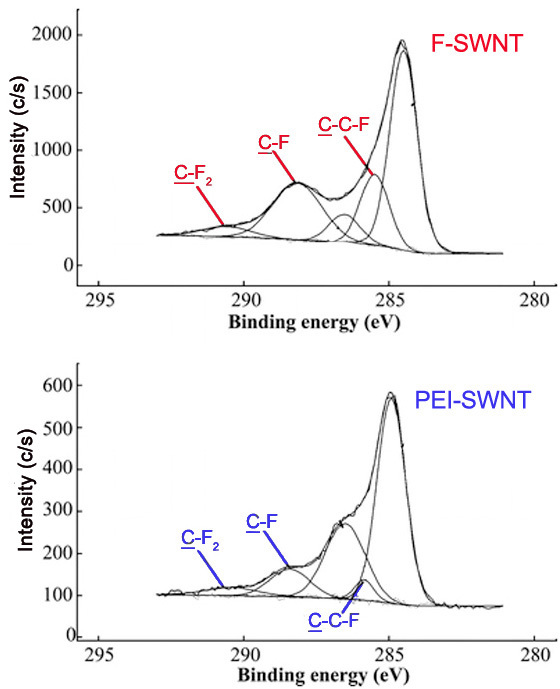
Comparing the relative intensities of various C1s peaks can be powerful in verifying that a reaction has occurred. Fluorinated carbon materials are often used as precursors to a broad range of variously functionalized materials. Reaction of fluorinated SWNTs (F-SWNTs) with polyethyleneimine (PEI) leads to decreases in the covalent carbon-fluoride C1s peak, as well as the evolution of the amine C1s peak. These changes are observed in the C1s spectra of the two samples ( [link] ).
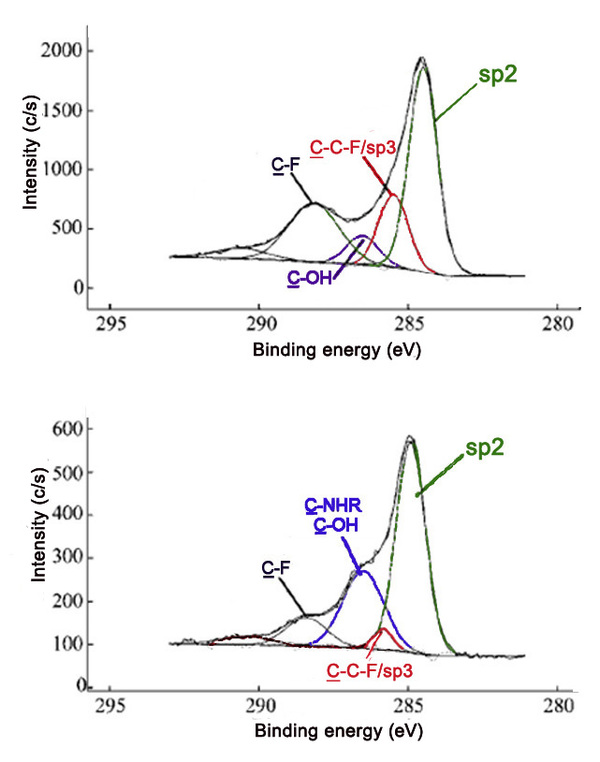
XPS can also be applied to determine the nature and extent of functionalization. In general, binding energy increases with decreasing electron density about the atom. Species with more positive oxidation states have higher binding energies, while more reduced species experience a greater degree of shielding, thus increasing the ease of electron removal.
The method of fluorination of carbon materials and such factors as temperature and length of fluorination affect the extent of fluoride addition as well as the types of carbon-fluorine bonds present. A survey scan can be used to determine the amount of fluorine compared to carbon. High resolution scans of the C1s and F1s peaks can also give information about the proportion and types of bonds. A shift in the peaks, as well as changes in peak width and intensity, can be observed in spectra as an indication of fluorination of graphite. [link] shows the Cls and F1s spectra of samples containing varying ratios of carbon to fluorine.

Furthermore, different carbon-fluorine bonds show characteristic peaks in high resolution C1s and F1s spectra. The carbon-fluorine interactions in a material can range from ionic to covalent. Covalent carbon-fluorine bonds show higher core electron binding energies than bonds more ionic in character. The method of fluorination affects the nature of the fluorine bonds. Graphite intercalation compounds are characterized by ionic carbon-fluorine bonding. [link] shows the F1s spectra for two fluorinated exfoliated graphite samples prepared with different methods.

Also, the peaks for carbons attached to a single fluorine atom, two fluorine atoms, and carbons attached to fluorines have characteristic binding energies. These peaks are seen in that C1s spectra of F- and PEI-SWNTs shown in [link] .
[link] lists various bonds and functionalities and the corresponding C1s binding energies, which may be useful in assigning peaks in a C1s spectrum, and consequently in characterizing the surface of a material.
| Bond/Group | Binding Energy (eV) |
| C-C | 284.0-286.0 |
| C-C (sp 2 ) | 284.3-284.6 |
| C-C (sp 3 ) | 285.0-286.0 |
| C-N | 285.2-288.4 |
| C-NR 2 (amine) | 285.5-286.4 |
| O=C-NH (amide) | 287.9-288.6 |
| -C = N (nitrile) | 266.3-266.8 |
| C-O | 286.1-290.0 |
| O=C-OH (carboxyl) | 288.0-289.4 |
| -C-O (epoxy) | 286.1-287.1 |
| -C-OH (hydroxyl) | 286.4-286.7 |
| -C-O-C- (ether) | 286.1-288.0 |
| -C=O (aldehyde/ketone) | 287.1-288.1 |
| C-F | 287.0-293.4 |
| -C-F (covalent) | 287.7-290.2 |
| -C-F (ionic) | 287.0-287.4 |
| C -C-F | 286.0-287.7 |
| C-F 2 | 291.6-292.4 |
| C-F 3 | 292.4-293.4 |
| C-S | 285.2-287.5 |
| C-Cl | 287.0-287.2 |
X-ray photoelectron spectroscopy is a facile and effective method for determining the elemental composition of a material’s surface. As a quantitative method, it gives the relative ratios of detectable elements on the surface of the material. Additional analysis can be done to further elucidate the surface structure. Hybridization, bonding, functionalities, and reaction progress are among the characteristics that can be inferred using XPS. The application of XPS to carbon nanomaterials provides much information about the material, particularly the first few atomic layers, which are most important for the properties and uses of carbon nanomaterials.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?