| << Chapter < Page | Chapter >> Page > |
Xenon was discovered by William Ramsay ( [link] ) and Morris Travers ( [link] ) on July 12, 1898, shortly after their discovery of krypton and neon.
Radon was the fifth radioactive element to be discovered after uranium, thorium, radium and polonium. Discovered in 1900 by Friedrich Dorn ( [link] ) after he noticed that radium compounds emanate a radioactive gas that he named Radium Emanation ( Ra Em ). Prior to these experiments, in 1899, Pierre and Marie Curie ( [link] ) observed that the gas emitted by radium remained radioactive for a month. Later that year, Ernest Rutherford ( [link] ) noticed variations when trying to measure radiation from thorium oxide. In 1901, he demonstrated that the emanations are radioactive, but credited the Curies for the discovery of the element.


The abundance of the Noble gases is given in [link] .
| Element | Terrestrial abundance (ppm) |
| He | 8 x 10 -3 (Earth’s crust), 4 x 10 6 (sea water), 5 (atmosphere) |
| Ne | 70 x 10 -3 (Earth’s crust), 0.2 (sea water), 18 (atmosphere) |
| Ar | 1.2 (Earth’s crust), 0.45 (sea water), 0.93 x 10 4 (atmosphere) |
| Kr | 10 x 10 -6 (Earth’s crust), 80 x 10 -6 (sea water), 1 (atmosphere) |
| Xe | 2 x 10 -6 (Earth’s crust), 100 x 10 -6 (sea water), 90 x 10 -3 (atmosphere) |
The naturally abundant isotopes of the Group 18 elements are listed in [link] . All of the isotopes of radon are radioactive.
| Isotope | Natural abundance (%) |
| Helium-3 | 0.000137 |
| Helium-4 | 99.999863 |
| Neon-20 | 90.48 |
| Neon-21 | 0.27 |
| Neon-22 | 9.25 |
| Argon-36 | 0.337 |
| Argon-86 | 0.063 |
| Argon-40 | 99.600 |
| Krypton-78 | 0.35 |
| Krypton-80 | 2.25 |
| Krypton-81 | trace |
| Krypton-82 | 11.6 |
| Krypton-83 | 11.5 |
| Krypton-84 | 57 |
| Krypton-86 | 17.3 |
| Xenon-124 | 0.095 |
| Xenon-126 | 0.089 |
| Xenon-128 | 1.91 |
| Xenon-129 | 26.4 |
| Xenon-130 | 4.07 |
| Xenon-131 | 21.2 |
| Xenon-132 | 26.9 |
| Xenon-134 | 10.4 |
| Xenon-136 | 8.86 |
| Radon-222 | trace |
Unlike most elements, helium's isotopic abundance varies greatly by origin, due to the different formation processes. The most common isotope, 4 He, is produced on Earth by a decay of heavier radioactive elements. It was also formed in enormous quantities during the Big Bang .
Naturally occurring 40 K with a half-life of 1.25 × 10 9 years, decays to stable 40 Ar (11.2%) by electron capture and positron emission, and also to stable 40 Ca (88.8%) via beta decay. These properties and ratios are used to determine the age of rocks.
With a half-life of 230,000 years 81 Kr is used for dating 50,000 - 800,000 year old groundwater. 85 Kr is an inert radioactive noble gas with a half-life of 10.76 years. It is produced in nuclear bomb testing and nuclear reactors. 85 Kr is released during the reprocessing of fuel rods from nuclear reactors.
Helium is extracted by fractional distillation from natural gas, which contains up to 7% helium. Since helium has a lower boiling point than any other element, low temperature and high pressure are used to liquefy nearly all the other gases. The resulting helium gas is purified by successive exposures to lowering temperatures. A final purification step with activated charcoal results in 99.995% pure Grade-A helium.
Argon is produced industrially by the fractional distillation of liquid air, a process that separates liquid nitrogen, which boils at 77.3 K, from argon, which boils at 87.3 K and oxygen, which boils at 90.2 K. Xenon is obtained commercially as a byproduct of the separation of air into oxygen and nitrogen.
The physical properties of the Group 18 elements are given in [link] .
| Element | Mp (°C) | Bp (°C) |
| He | -272.20 | -268.93 |
| Ne | -248.59 | -246.08 |
| Ar | -189.35 | -185.85 |
| Kr | -157.36 | -153.22 |
| Xe | -111.7 | -108.12 |
| Rn | -71.15 | -61.85 |
All of the Noble gases show characteristic spectral lines ( [link] – [link] ).





Only a few hundred noble gas compounds have been formed. Neutral compounds of helium and neon have not been formed, while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order:
Xenon compounds are the most numerous of the noble gas compounds. Oxidation states of +2, +4, +6, and +8 with electronegative elements, e.g., XeF 2 , XeF 4 , XeF 6 , XeO 4 , and Na 4 XeO 6 . Compounds of xenon bound to boron, hydrogen, bromine, iodine, beryllium, sulphur, titanium, copper, and silver have also been observed but only at low temperatures in noble gas matrices, or in supersonic noble gas jets.
Although radon is more reactive than xenon it should form chemical bonds more easily than xenon, however, due to the high radioactivity and short half-life of radon isotopes, only a few fluorides and oxides of radon have been formed.
Krypton is less reactive than xenon, and oxidation states are generally limited to +2, KrF 2 . Compounds in which krypton forms a bond to nitrogen and oxygen are only stable below -60 °C and -90 °C, respectively. Krypton atoms chemically bound to other nonmetals (hydrogen, chlorine, carbon) as well as some late transition metals (copper, silver, gold), but only at low temperatures in noble gas matrices, or in supersonic noble gas jets. Similar conditions were used to obtain the first compounds of argon.
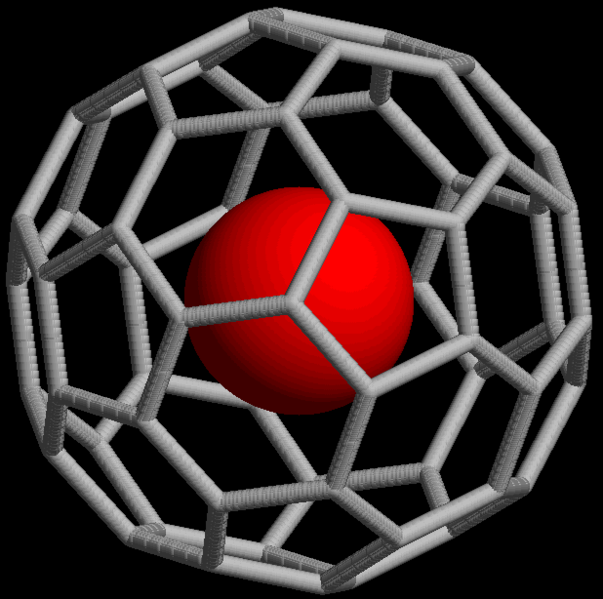
Noble gases also form non-covalent compounds, for example clathrates that consist of an atom trapped within cavities of crystal lattices of organic and inorganic compounds. Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule ( [link] ).

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?