| << Chapter < Page | Chapter >> Page > |
Organisms rely on nutrients in order to survive. These include carbon, oxygen, nitrogen, water and mineral salts.
These nutrients need to be cycled through the ecosystem so that they can be re- used. This is called nutrient recycling . In biology, this is the movement of nutrients from the physical environment into living organisms and back into the environment. The flow of energy you sawbefore from the sun to herbivores and then to carnivores is part of this process. In any environment the flow of nutrients must be stable and balancedso that organisms can survive. If the cycle stops at any point, nutrients will become locked in place and cannot be used in the next step.
The water cycle, carbon cycle, oxygen cycle and nitrogen cycle are examples of nutrient re-cycling.
Video: (External Link)
Here is a simple video explaining nutrient cycling
(ex (External Link) )
The earth is sometimes known as the "water planet" because over 70 percent of its surface is covered by water. All living organisms need water for theirsurvival.
In this cycle, water is transported between water reservoirs in the environment and living organisms. This happens through these processes:

Animation: (External Link)
This is an animation of the water cycle
Oxygen is one of the main gases found in the air, along with nitrogen.
Oxygen is re-cycled between the air and living organisms in the following ways:
Because animals trap oxygen during respiration, the release of oxygen by plants during photosynthesis is the main way oxygen is released into the atmosphere.

Video: (External Link)
This is a video explaining the oxygen cycle
Carbon is the basic building block of all organic materials, and therefore, of living organisms. Most of the carbon on earth can be found in the crust. Otherreservoirs of carbon include the oceans and atmosphere.
Carbon moves from one reservoir to another by these processes:
Photosynthesis and respiration are the main carbon cycling processes involving living organisms.

GAME: (External Link)
This is a game you can play to learn more about the carbon cycle
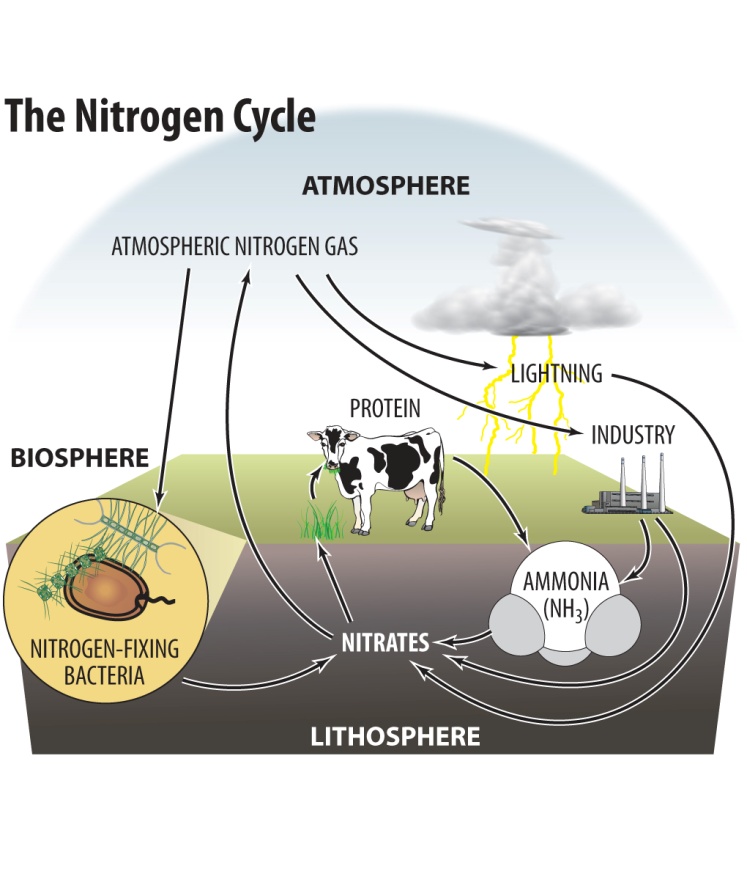
Nitrogen (N2) makes up most of the gas in the atmosphere (about 78%). Nitrogen is important to living organisms and is used in the production of amino acids,proteins and nucleic acids (DNA, RNA). Only a few single-cell organisms, like bacteria can use nitrogen from the atmosphere directly. For multi-cellularorganisms, like plants and animals, nitrogen has to be changed into other forms, eg. nitrates or ammonia. This process is known as nitrogen fixation .
The nitrogen cycle involves these steps:
During decomposition , bacteria and fungi break down proteins and amino acids from plants and animals into nitrogen in the form of ammonia (NH3) by the process of ammonification and convert the ammonia to nitrate (NO3-) by nitrification .
Nitrogen can be changed to nitrates directly by lightning . The rapid growth of fungi and algae after thunderstorms is because of this process, which increases the amount of nitrates that fall onto the earth inrain water, acting as fertilizer.
Ammonia and nitrates are absorbed by plants through their roots.
Humans and animals get their nitrogen supplies by eating plants or plant-eating animals.
The nitrogen is returned to the cycle when bacteria decompose the waste or dead bodies of these higher organisms, and in the process, convert organic nitrogen into ammonia.
In a process called denitrification , other bacteria convert ammonia and nitrate into nitrogen and nitrous oxide (N 2 O). Nitrogen is returned to the atmosphere to start the cycle over again.
SIMULATION: http://www.teachersdomain.org/asset/lsps07_int_nitrogen/
You can play with this simulation to learn more about the Nitrogen cycle.
Animation:
Here are some animations of the nitrogen cycle:

Notification Switch
Would you like to follow the 'Siyavula: life sciences grade 10' conversation and receive update notifications?