| << Chapter < Page | Chapter >> Page > |
Die planeet Aarde word deur ‘n kombers van gasse bedek wat ons die atmosfeer noem. Die dikte van die laag (atmosfeer) teenoor die grootte van die aarde, kan met die skil van ‘n lemoen teenoor die grootte van die lemoen vergelyk word.

Sonder die atmosfeer sou lewe op aarde nie moontlik wees nie. Dit bestaan nie alleen uit gasse wat noodsaaklik is vir lewe nie, maar verhoed ook dat ons deur die son “gebraai” of deur die koue van die buitenste ruim “verkluim” word. Die atmosfeer veroorsaak dat slegs die helfte van die son se strale die oppervlak van die aarde bereik.
Ons onderskei tussen vier lae in die atmosfeer .

Laag 1: die troposfeer
Hierdie is die laag teenaan die aardoppervlak. Dit is ongeveer 11 km dik en dit is in hierdie laag waar weerveranderinge in die lug plaasvind. Water kom feitlik uitsluitlik in hierdie laag voor.
Die lug in die troposfeer bestaan hoofsaaklik uit twee gasse: stikstof en suurstof. Daar is ‘n baie klein hoeveelheid ander gasse.
Jy kan die samestelling van die lug met ballonne voorstel:
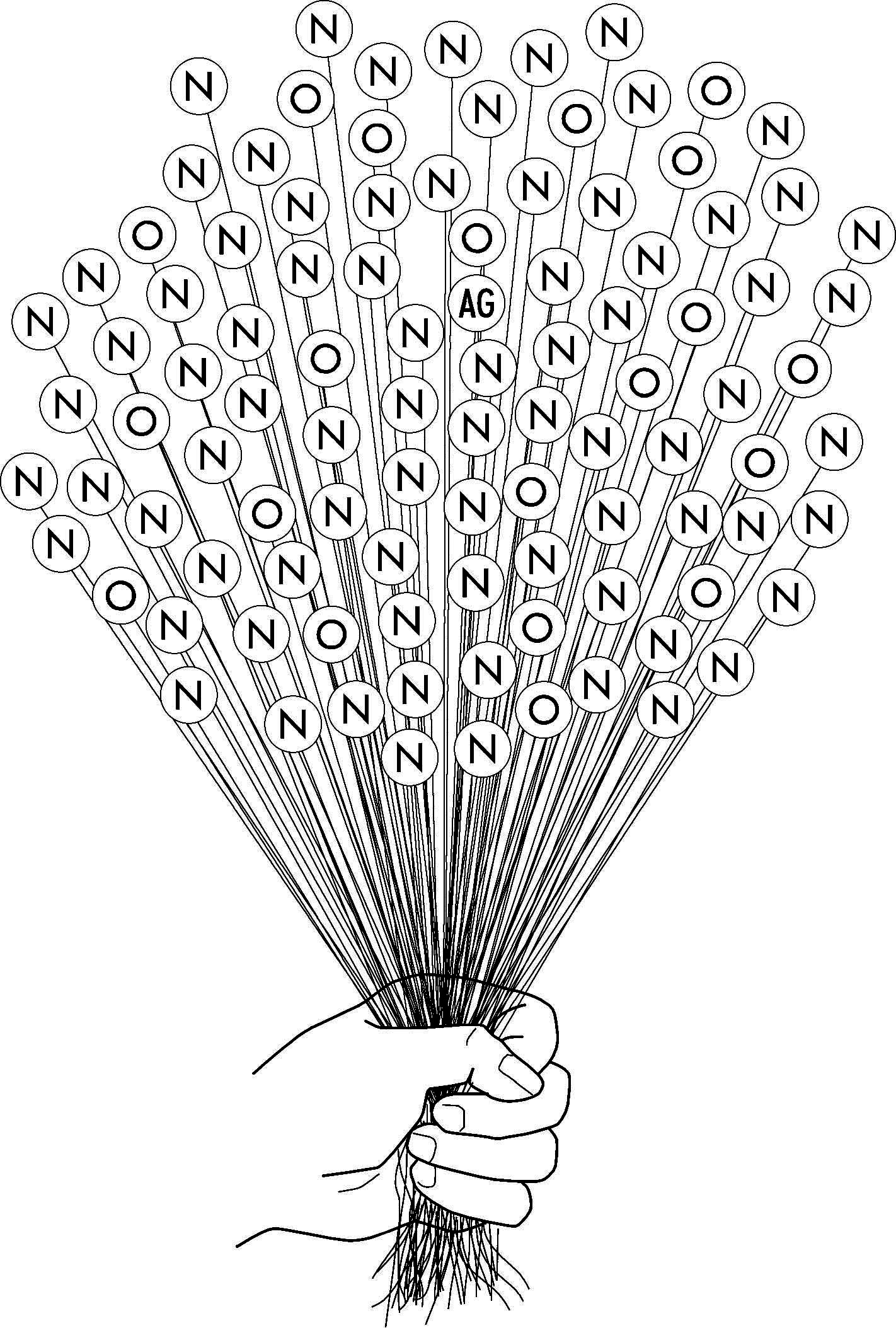
Tel die ballonne en skryf die getalle neer:
Die met ‘n N (dit staan vir stikstof): _______________________________
Die met ‘n O (dit staan vir suurstof): _______________________________
Die met ‘n AG (dit staan vir ander): _______________________________
Beskryf nou in jou eie woorde hoe die lug in die troposfeer saamgestel is (die lug wat ons inasem):
Hoe hoër mens in die troposfeer opbeweeg, hoe “dunner” word die lug. Dit beteken die deeltjies is verder uitmekaar versprei. As jy byvoorbeeld op ‘n hoogte van 8 km ‘n houer met lug sou vul, sal daar minder deeltjies (stikstof, suurstof en ander gasse) in die houer wees as wanneer jy dit op die grond sou doen.
Laag 2: die stratosfeer
Die stratosfeer is ongeveer 40 km dik. Vliegtuie vlieg in hierdie laag. Die baie belangrike osoonlaag kom hier voor. Dit is ‘n tipe suurstoflaag wat die aarde teen die nadelige strale van die son beskerm. Sonder hierdie laag sal die meeste lewende wesens op aarde sterf. Die suurstof in die osoonlaag kom vanaf die plante wat op aarde groei. As hierdie balans versteur word deurdat minder suurstof die osoonlaag bereik en die plek daarvan deur ander gasse wat deur sekere menslike aktiwiteite vrygestel word opgeneem word, verander dit die temperatuur op aarde. Dit kan uiteindelik meebring dat dier en plant nie meer sal kan oorleef nie.
Laag 3: die mesosfeer
Hierdie laag is ongeveer 40 km dik en word gekenmerk deur temperatuurwisseling vanaf -113 grade Celsius tot by 440 grade Celsius (jou liggaamstemperatuur is 37 grade Celsius, water vries by ongeveer 0 grade Celsius en kook by ongeveer 100 grade Celsius). Vallende rotsstukke uit die buiteruim verbrand in hierdie laag en ons sien dit as “verskietende sterre”.
Laag 4: die ionosfeer (termosfeer)
Die ionosfeerlaag is ongeveer 350 km dik en bevat baie gelaaide deeltjies (ione) wat maak dat baie van die radioaktiwiteit wat by tye deur die son veroorsaak word, uitgeskakel word en nie ‘n effek op die aarde het nie.
Vrae:
1. Hoekom is dit belangrik dat ons plante plant eerder as om hulle uit te roei?
2. Industriële vooruitgang kan nadelig wees vir die natuur omdat dit die atmosfeer kan verander. Verduidelik hoekom.
3. Hoekom moet bergklimmers wat hoë berge soos Everest klim, suurstofmaskers dra?
LU 2
KONSTRUKSIE VAN WETENSKAPKENNIS Die leerder ken, interpreteer en pas wetenskaplike, tegnologiese en omgewingskennis toe.
Dit is duidelik wanneer die leerder:
2.1 betekenisvolle inligting onthou;
2.2 inligting kategoriseer en eie reëls vir kategorisering verduidelik.
Getal ballonne (laat egter eers die leerders self tel):
Samestelling: In elke 100 dele is daar 79 dele stikstof (79%), 20 dele suurstof (20 dele) en een deel ander gasse (1%)
Antwoorde:
L.W. Hierdie vrae moet spesifiek t.o.v. die atmosfeer beantwoord word.
3. Die druk raak so laag dat daar te min suurstof per volume eenheid is.

Notification Switch
Would you like to follow the 'Natuurwetenskappe graad 5' conversation and receive update notifications?