| << Chapter < Page | Chapter >> Page > |
Groupwork:
Observation: The glowing wooden splinter catches fire because oxygen has been released.
Conclusion: Mercury oxide is a compound that decomposed as a result of heating. It broke up into oxygen (a gas) and mercury (a metal).
Mercury Oxide = mercury + oxygen
Group work:
Do the following experiment in your groups to determine whether mercuric oxide is a compound consisting of elements. Follow the different steps exactly:
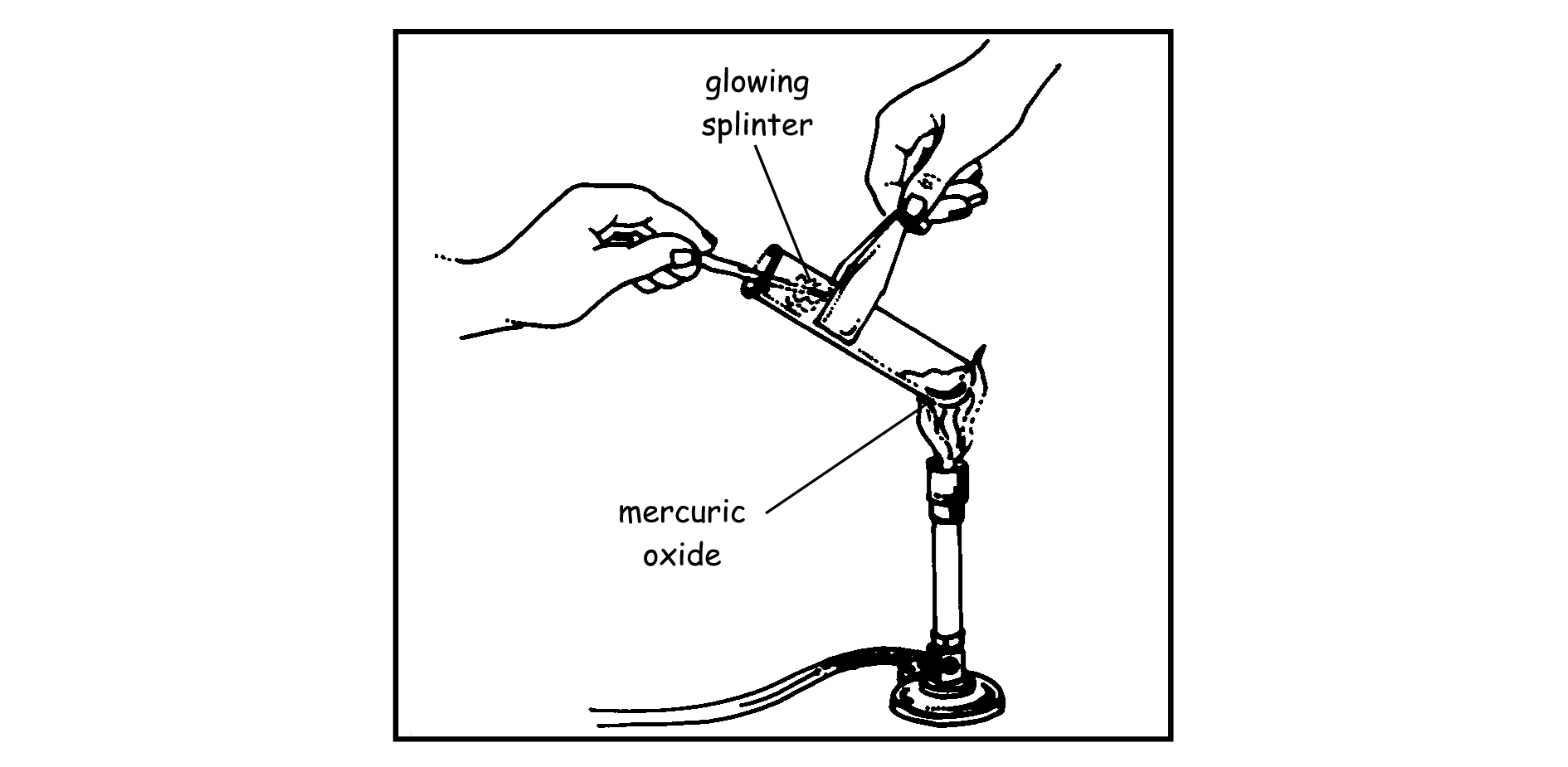
Observation: _________________________________________________________
____________________________________________________________________
____________________________________________________________________
Deduction: __________________________________________________________
____________________________________________________________________
____________________________________________________________________

Assessment
| MARK | LEVEL | COOPERATIVELEARNING SKILLS | VALUES AND ATTITUDES | CONTENT |
| 1-34% | 1 | Each learner learns individually. Participation in the group is destructive, e.g. domineering. | No respect for others. Destructive interpersonal relationships (actively negative). | Not able to come to meaningful observations and deductions. |
| 35-39 % | 2 | Does not contribute to the group and forms no meaningful part of it. | Not respectful towards others, but this does not affect interpersonal relationships (negatively passive). | Observations and deductions fairly sound. |
| 40-69% | 3 | Acknowledged as a member of the group. Sensitive to the needs of others. | Shows consideration for others and understanding of different points of view, but this has no effect on interpersonal relationships (positively passive). | Observations and deductions sound. |
| 70-100% | 4 | Creates opportunities for contributions by other members of the group. Always contributes positively to the group task. | Shows consideration for others and understanding of different points of view. Influences interpersonal relationships in a positive way (positively active). | Observations and deductions excellent. |
Learning Outcome 1: The learner will be able to act confidently on curiosity about natural phenomena, and to investigate relationships and solve problems in scientific, technological and environmental contexts.
Assessment Standard 1.2: We know this when the learner conducts investigations and collects data: organises and uses apparatus/equipment or sources to gain and record information;
Assessment Standard 1.3: We know this when the learner evaluates data and communicates findings: generalises in terms of relevant aspects and describes how the data support the generalisation.

Notification Switch
Would you like to follow the 'Natural sciences grade 7' conversation and receive update notifications?