| << Chapter < Page | Chapter >> Page > |
Group 13 halides are used as synthons for their organometallic derivatives, [link] and [link] .
All of the trihalides are strong Lewis acids, and as such react with Lewis base compounds to form Lewis acid-base complexes, [link] . The extent of the equilibrium is dependant on the Lewis acidity of the trihalide and the basicity of the Lewis base. For example, with BCl 3 , oxygen donor ligands result in approximately 50:50 ratio of BCl 3 and BCl 3 L, while for nitrogen donor ligands the equilibrium is shifted to the formation of the complex.
The general structure of the Lewis acid-base complexes is such that the Group 13 element is close to tetrahedral ( [link] ). However, for aluminum and the heavier Group 13 elements, more than one ligand can coordinate ( [link] ) up to a maximum of six.
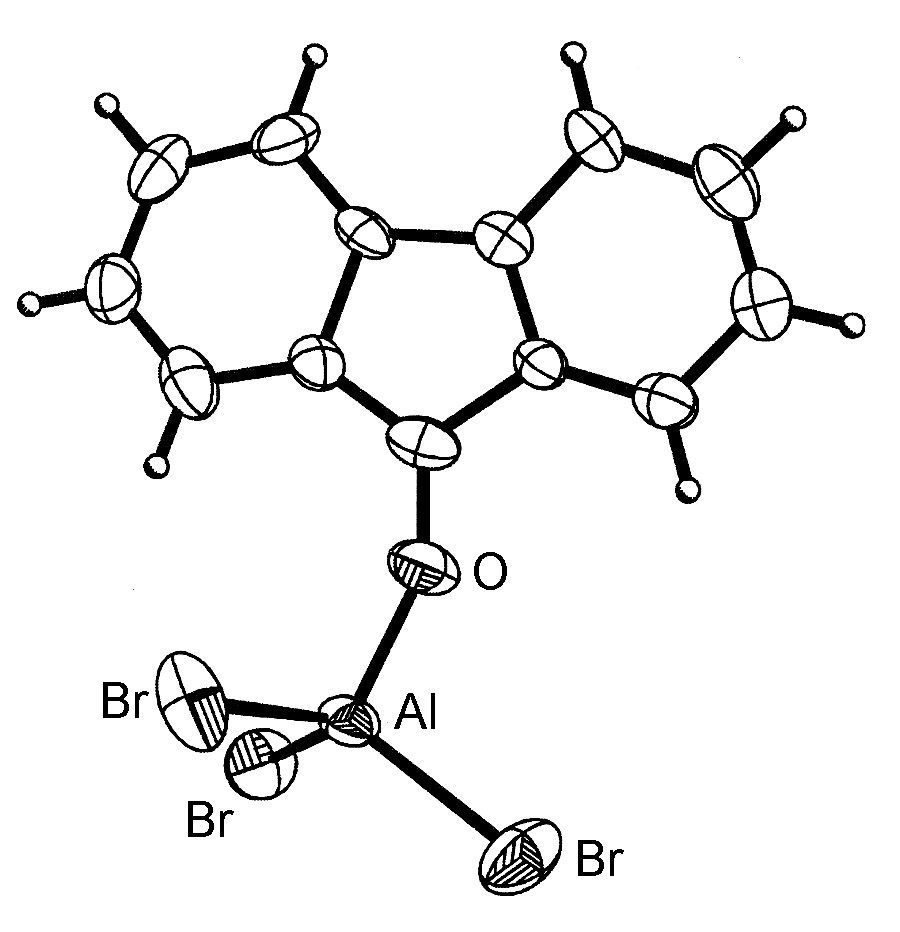

It should be noted that the dimeric form of MX 3 ( [link] ) can be thought of as mutual Lewis acid-base complexes, in which a Lewis basic lone pair of a halide on one MX 3 unit donates to the Lewis acidic metal on another MX 3 unit.
Generally the fluorides are insoluble in water while the heavier halides are more soluble. However, BF 3 , BCl 3 , and BBr 3 all decompose in the presence of water, [link] . In the case of the fluoride, the HF formed reacts with BF 3 to form fluoroboric acid, [link] . However, there is also a minor equilibrium (2-3%) resulting in the formation of the BF 3 complex of OH - and H 3 O + , [link] .
While the boron compounds (and AlBr 3 ) decompose even in moist air, AlCl 3 reacts more slowly to make aluminum chlorohydrate (ACH) which has the general formula Al n Cl 3n-m (OH) m . While ACH has been proposed to exist as a number of cluster species, it is actually a range of nanoparticles.
ACH is also known as polyaluminum chloride (PAC). The latter name is often used in water purification, where ACH is preferred over alum derivatives (Al 2 (SO 4 ) 3 ). The combination of ACH and a high molecular weight quaternized ammonium polymer (e.g., dially dimethyl ammonium chloride (DADMAC)), has been known as an effective combination as a flocculant in water treatment process to remove dissolved organic matter and colloidal particles present in suspension.
Aluminum chlorohydrate (ACH) and aluminum-zirconium compounds, are frequently used as the active ingredient in antiperspirants. The mode of action of most aluminum-based compounds involves the dramatic change in the particle size from nano to micro as a function of pH and electrolyte changes on the skin (as compared to the antiperspirant stick or suspension) and hence forming a gel plug in the duct of the sweat gland. The plugs prevent the gland from excreting liquid and are removed over time by the natural sloughing of the skin. A further mechanism of action involves the absorption of 3-methyl-2-hexenoic acid ( [link] ). Human perspiration is odorless until bacteria ferment it. Bacteria thrive in hot, humid environments such as the underarm. When adult armpits are washed with alkaline pH soaps, the skin loses its acid mantel (pH = 4.5 - 6), raising the skin pH and disrupting the skin barrier. The bacteria thrive in the basic environment, and feed on the sweat from the apocrine glands and on dead skin and hair cells, releasing 3-methyl-2-hexenoic acid, which is the primary cause of body odor. As with all carboxylic acids, 3-methyl-2-hexenoic acid, reacts in a facile manner with the surface of the alumina nanoparticles. Aluminum chloride salts also have a slight astringent effect on the pores; causing them to contract, further preventing sweat from reaching the surface of the skin.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?