| << Chapter < Page | Chapter >> Page > |
When a chemical change takes place, new substances are formed in a chemical reaction. These new products may have very different properties from the substances that were there at the start of the reaction.
The breakdown of copper(II) chloride to form copper and chlorine is an example of chemical change. A simplified diagram of this reaction is shown in [link] . In this reaction, the initial substance is copper(II) chloride, but once the reaction is complete, the products are copper and chlorine.

The formation of new substances in a chemical reaction. One type of matter is changed into something different.
There are some important things to remember about chemical changes:
We will consider two types of chemical reactions: decomposition reactions and synthesis reactions .
A decomposition reaction occurs when a chemical compound is broken down into elements or smaller compounds. The generalised equation for a decomposition reaction is:
AB A + B
One example of such a reaction is the decomposition of hydrogen peroxide ( [link] ) to form hydrogen and oxygen according to the following equation:
2H 2 O 2 2H 2 O O 2
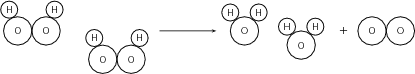
The decomposition of mercury (II) oxide is another example.
Aim
To observe the decomposition of mercury (II) oxide when it is heated.
Note: Because this experiment involves mercury, which is a poisonous substance, it should be done in a fume cupboard, and all the products of the reaction must be very carefully disposed of.
Apparatus
Mercury (II) oxide (an orange-red product); two test tubes; a large beaker; stopper and delivery tube; Bunsen burner; wooden splinter.

Method

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science' conversation and receive update notifications?