| << Chapter < Page | Chapter >> Page > |
Tellurites can be extracted from the mixture with water and are normally present as hydrotellurites HTeO 3 - in solution.Selenates are also formed during this process, but they can be separated by adding sulfuric acid. The hydrotellurites are converted into the insoluble tellurium dioxide while the selenites stay in solution, [link] .
The reduction to the metal is done either by electrolysis or by reacting the tellurium dioxide with sulfur dioxide in sulfuric acid, [link] .
The physical properties of the Group 16 elements encompasses a gas (O 2 ), a non-metallic solid (S 2 ), and metals (Se, Te, Po), [link] .
| Element | Mp (°C) | Bp (°C) | Density (g/cm 3 ) |
| O | -218.79 | -182.95 | 1.429 g/L |
| S | 115.21 | 444.6 | 1.819 |
| Se | 221 | 685 | 4.81 (gray), 4.39 (alpha), 4.28 (vitreous) |
| Te | 449.51 | 988 | 6.24 (solid), 5.70 (liquid) |
| Po | 254 | 962 | 9.196 (alpha), 9.398 (beta) |
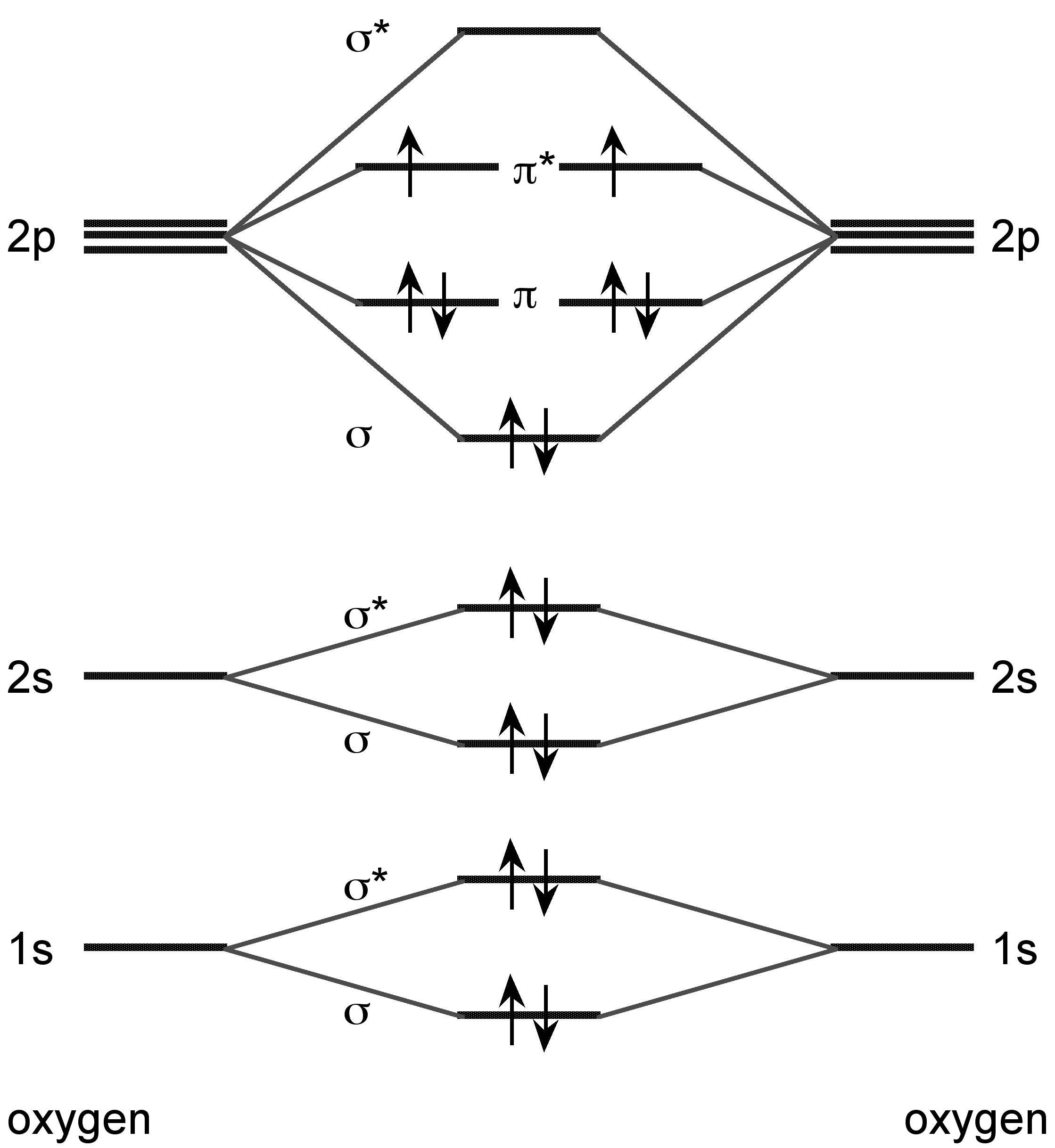
The lighter Group 16 elements form X 2 dimers in the vapor phase. Sulfur also forms higher allotropes in the vapor phase (e.g., S 8 and S 6 ), while selenium and tellurium forms atomic vapor in addition to the dimmers. Unlike dihydrogen, however, the bonding is associated with the molecular orbital combination of the two π-orbitals ( [link] ). All of the dimeric X 2 molecules are paramagnetic.
While sulfur forms over 30 allotropes, the common form of sulfur is cyclooctasulfur (S8) has three main allotropes: Sα, Sβ, and Sγ. The orthorhombic form (Sα) is more stable up to 95 °C, while the β-form is the thermodynamic form. The lone pairs of electrons make the S-S-S bend (108°), resulting in S 8 having the shape of a crown ( [link] ). When sulfur melts the S 8 molecules break up. When suddenly cooled, long chain molecules are formed in the plastic sulfur that, behave as rubber. Plastic sulfur transform into rhombic sulfur over time.

Elemental selenium produced in chemical reactions appears as the amorphous red form. When rapidly melted, it forms the black, vitreous form, which is usually sold industrially as beads. The most thermodynamically stable and dense form of selenium is the electrically conductive gray (trigonal) form, which is composed of long helical chains of selenium atoms ( [link] ). The conductivity of gray selenium is light sensitive and is hence used in photocopiers. Selenium also exists in three different deep-red crystalline monoclinic forms, which are composed of Se8 molecules, similar to many allotropes of sulfur. Unlike sulfur, however, selenium does not undergo the changes in viscosity when heated.
Tellurium is a crystalline metal with a triginal structure ( a = 4.4572 Å, c = 5.929 Å). Polonium has a simple cubic structure in it’s a form ( a = 3.359 Å)
The chemistry of the Group 16 elements is dominated by the stability of the -2 oxidation state and the noble gas configuration of the X 2- anion.
The electronegativity of oxygen (3.5) results in it having predominantly -2 oxidation state, however, sulfur, selenium and tellurium all for compounds with higher oxidation states, especially with oxygen ( [link] ).
| Element | -2 | -1 | +4 | +6 |
| O | Na 2 O, H 2 O | H 2 O 2 | - | - |
| S | H 2 S | H 2 S 2 | SO 2 | H 2 SO 4 , SO 3 |
| Se | H 2 Se | H 2 Se 2 | SeO 2 | SeF 4 |
| Te | H 2 Te | t Bu 2 Te 2 | TeO 2 | Te(OH) 6 |
Catenation is the ability of a chemical element to form a long chain-like structure via a series of covalent bonds. Although oxygen shows this property only in the existence of ozone, sulfur is second only to carbon in exhibiting this mode of combination; the chalcogens beyond sulfur show it to diminishing degrees, polonium having no tendency to catenate.
When aqueous metal sulfide salts are heated with elemental sulfur a range of polysulfide ions are formed, [link] . When alkali polysulfides dissolve in polar solvents (e.g., DMF or DMSO) a deep blue solution is formed. The absorption (λ max = 610 nm) is associated with the radical anion, S 3 - . While, polyselenides and polytellurides are less common, the Se 3 2- and Te 3 2- ions are known.
The term polysulfide often refers to a class of polymers with alternating chains of several sulfur atoms and hydrocarbon substituents. The general formula is R 2 Sn, where n ranges from 2 – 10. For the selenium and tellurium analogs the extent of catenation is far more limited.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?