| << Chapter < Page | Chapter >> Page > |
| Compound | Molecular weight (g/mol) | Mpt (°C) | Bpt (°C) |
| H 2 O | 18.01 | 0 | 100 |
| H 2 S | 34.08 | -85.5 | -60.7 |
| H 2 Se | 80.98 | -60.4 | -41.5 |
| H 2 Te | 129.62 | -49 | -2 |
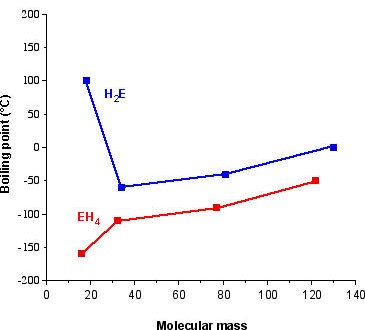
A similar but not as pronounced trend is observed for the Group 15 hydrides, where ammonia’s higher values are associated with the presence of significant hydrogen bonding ( [link] ).
| Compound | Molecular weight (g/mol) | Mpt (°C) | Bpt (°C) |
| NH 3 | 17.03 | -77.7 | -33.35 |
| PH 3 | 34.00 | -133.5 | -87.4 |
| AsH 3 | 77.95 | -113.5 | -55 |
| SbH 3 | 124.77 | -88.5 | -17 |
Would you expect H 2 S 2 to have a higher or lower boiling point that H 2 O 2 ? Why?
The boiling point for H 2 O 2 is 150.2 °C, while that of 70.7 °C. The difference in boiling point is due to the stronger intermolecular hydrogen bonding in H 2 O 2 than in H 2 S 2 .
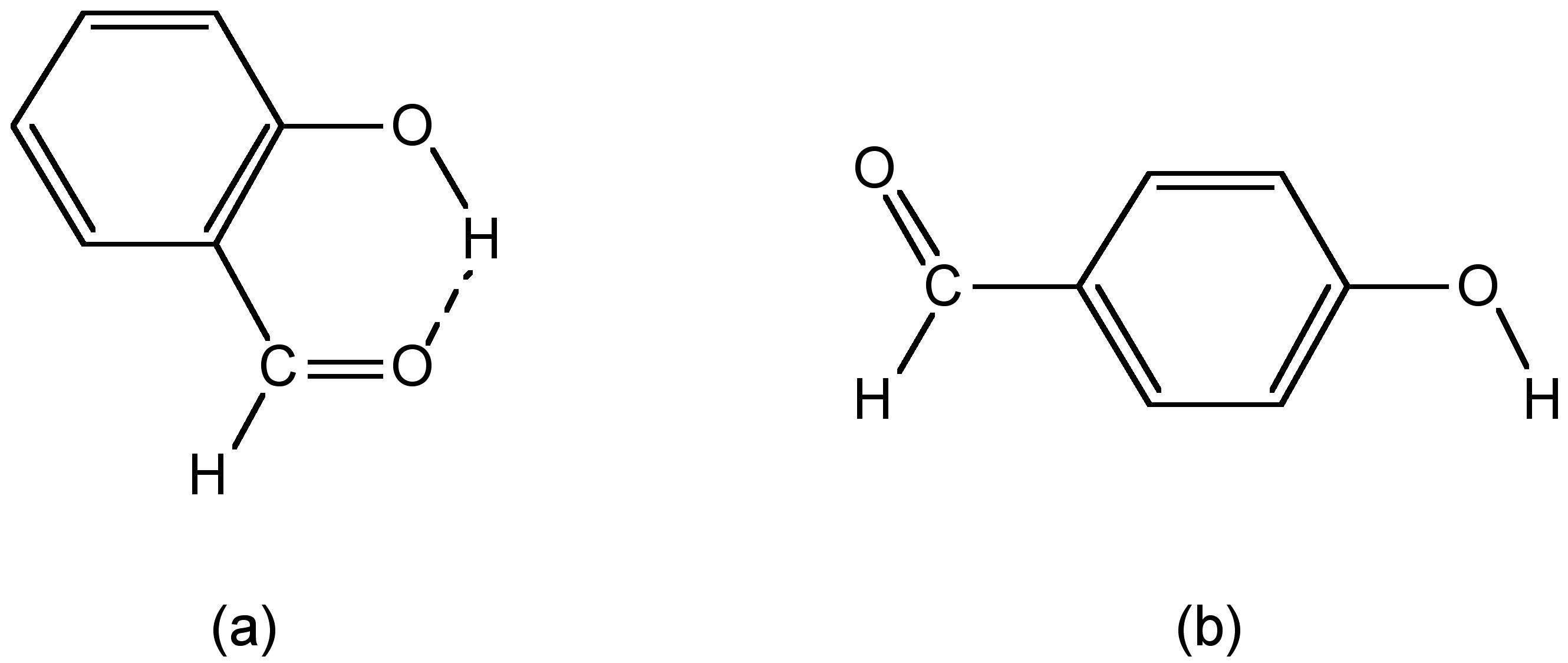
The types of hydrogen bond can also have a significant effect on the physical properties of a compound. For example, the cis isomer of hydroxybenzaldehyde melts at 1 °C, while the trans isomer has melting and boiling points of 112 °C. Both compounds exhibit strong hydrogen bonding in the solid state, however, as may be seen from [link] a, cis -hydroxybenzaldehyde (salicylaldehyde) has a configuration that allows strong intramolecular hydrogen bonding, which precludes any intermolecular hydrogen bonding. The melting point of cis -hydroxybenzaldehyde is going to be controlled by the van der Waal forces between adjacent molecules. In contrast, since intramolecular hydrogen bonding is precluded in the trans isomer ( [link] b) it can form strong intermolecular hydrogen bonds in the solid state, and thus, it is these that define the melting point. The boiling points are controlled in a similar manner.
4-Hydroxybenzoic acid melts at 213 °C, while 2-hydroxybenzoic acid melts at 158 °C. Explain this observation.
2-Hydroxybenzoic acid exhibits strong intramolecular hydrogen bonding while 4-hydroxybenzoic acid has strong intermolecular hydrogen bonding.
Melting and boiling are not the only physical properties that are affected by hydrogen bonding. Solubility can also be affected. Consider two isomers of C 4 H 10 O: n BuOH and Et 2 O. The n-butanol is much more soluble in water than diethyl ether. The reason for this is that while both compounds can hydrogen bond to water, those between n BuOH and water are much stronger than those between Et 2 O and water, and thus, dissolution of n BuOH in water does not disrupt the very strong hydrogen bonding in water as much as Et 2 O does.
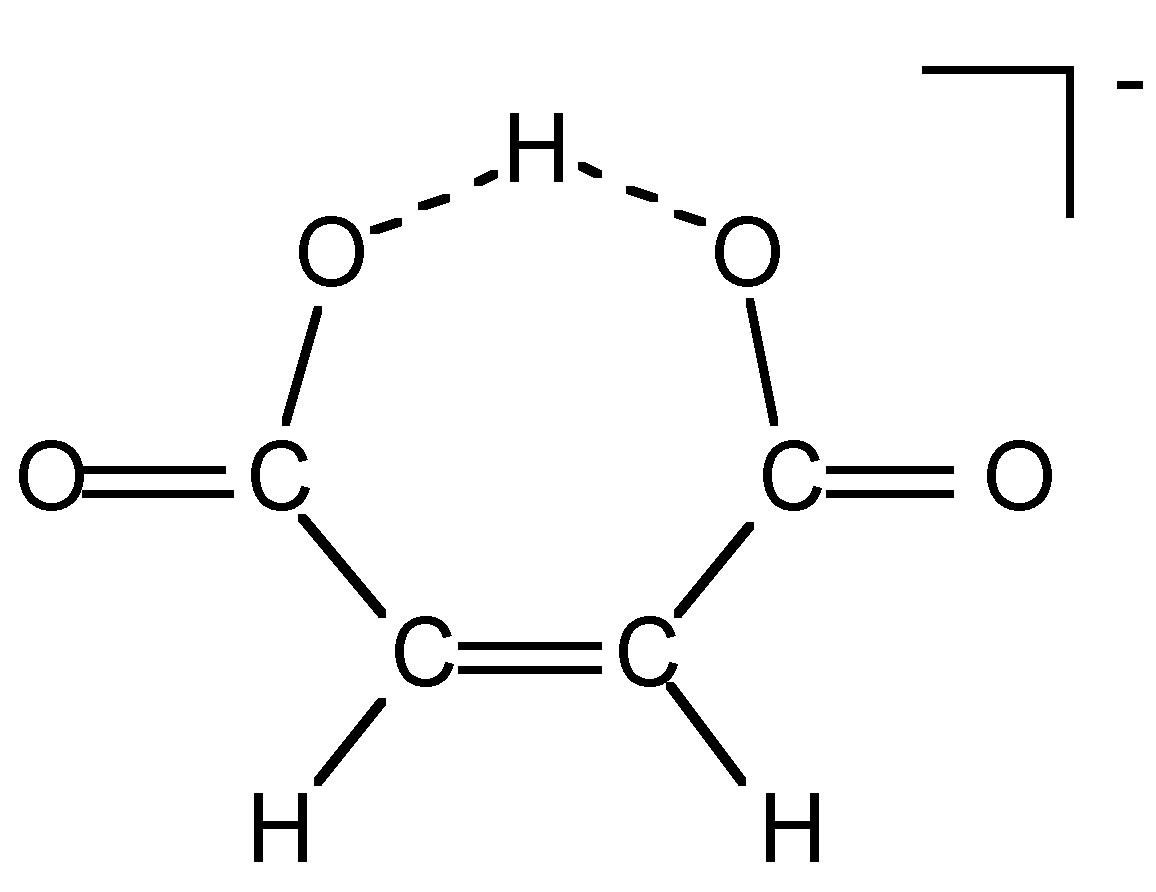
The acidity of a protic species can be affected by the presence of hydrogen bonding. For example, consider the di-carboxylic acid derivatives of ethylene (fumaric acid). Each of the carboxylic acid groups has sequential equilibria that may be defined by the pK values, [link] . [link] lists the pK values for the cis and trans isomers. The acidity of the first and second carboxylic group for the trans isomer is similar, the difference being due to the increased charge on the molecule. In contrast, the second proton in the cis isomer is much less acidic than the first proton: why? A consideration of the structure of the mono anion of the cis isomer ( [link] ) shows that a very strong intramolecular hydrogen bond is formed once the first proton is removed. This hydrogen bond makes the second acidic proton much more difficult to remove and thus lowers the acidity of the proton.
| Isomer | pK 1 | pK 2 | Ratio |
| Trans | 3 | 4.5 | 25:1 |
| Cis | 1.9 | 6.2 | 10,000:1 |
In the solid state, molecules that can form hydrogen bonds will tend to arrange themselves so as to maximize the formation of linear hydrogen bonds. The structure of ice is a typical example, where each water molecule’s hydrogen bonds to four other molecules creating a diamond-like lattice ( [link] ). However, ice isn’t the only compound whose solid state structure is defined by its hydrogen bonding. Cyanuric acid forms six strong intermolecular hydrogen bonds to three other molecules in the plane of the heterocyclic ring and thus creates a graphite-like structure ( [link] ).


The presence of hydrogen bonding can stabilize certain conformations over others that in the absence of hydrogen bonding would be more favored. For example, based on steric considerations H 2 NCH 2 CH 2 C(O)H would be expected to adopt a staggered conformation as is typical for compounds with free rotation about a C-C bond. However, due to the presence of a strong intramolecular hydrogen bond it adopts a sterically disfavored eclipsed conformation ( [link] ).

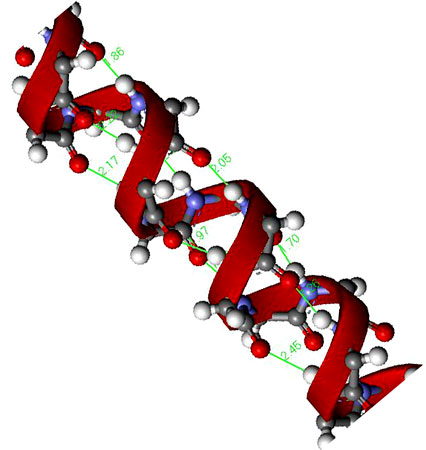
This conformational stabilization is even more important in biopolymers such as peptides. If we assume that a peptide chain ( [link] ) made from a sequence of amino acids would not adopt any eclipsed conformations on steric grounds, and that the amide group (H-N-C=O) is near planar due to delocalization, then there will be 3 2 possible conformations per nitrogen in the peptide chain, i.e., three each for the C-C and C-N bonds ( [link] ). Assuming a modest peptide has 50 amino acids, there will be 3 2x50 potential conformations of the polymer chain, i.e., over 5 x 10 47 conformations. However, in reality there are limits to the number of conformations observed since particular ones are stabilized by intramolecular hydrogen bonds. The most important of these are the α-helix ( [link] ) and β-sheet structures.


Notification Switch
Would you like to follow the 'Hydrogen' conversation and receive update notifications?