| << Chapter < Page | Chapter >> Page > |

After ca. 3 hours of hydration, the rate of C–S–H formation increases with the amount of C–S–H formed. Solidification of the paste, called setting, occurs near the end of the third period. The fourth stage ( [link] d) is the deceleratory period in which hydration slowly continues hardening the solid cement until the reaction is complete. The rate of hydration in this phase is determined either by the slow migration of water through C–S–H to the inner, unhydrated regions of the particles, or by the migration of H + through the C–S–H to the anhydrous CaO and SiO 2 , and the migration of Ca 2+ and Si 4+ to the OH - ions left in solution.
In spite of the fact that the aluminate and ferrite phases comprise less than 20% of the bulk of cement, their reactions are very important in cement and dramatically affect the hydration of the calcium silicate phases, see below. Relative to C3S, the hydration of C3A is very fast. In the absence of any additives, C3A reacts with water to form two intermediate hexagonal phases, C2AH8 and C4AH13, [link] . The structure of C2AH8 is not precisely known, but C4AH13 has a layered structure based on the calcium hydroxide structure, in which one out of every three Ca 2+ is replaced by either an Al 3+ or Fe 3+ with an OH - anion in the interlayer space to balance the charge. All of the aluminum in C4AH13 is octahedral. C2AH8 and C4AH13 are meta-stable phases that spontaneously transform into the fully hydrated, thermodynamically stable cubic phase, C3AH6, [link] . In C3A, aluminum coordination is tetrahedral. The structure consists of rings of aluminum tetrahedra linked through bridging oxygen atoms, which slightly distorts the aluminum environment. In C3AH6, aluminum exists as highly symmetrical, octahedral Al(OH) 6 units.
If the very rapid and exothermic hydration of C3A is allowed to proceed unhindered in cement, then the setting occurs too quickly and the cement does not develop strength. Therefore, gypsum [calcium sulfate dihydrate, CaSO 4 ⋅2(H 2 O)] is added to slow down the C3A hydration. In the presence of gypsum, tricalcium aluminate forms ettringite, [Ca 3 Al(OH) 6 .12(H 2 O)] 2 .(SO 4 ) 3 .2(H 2 O), [link] , which can also be written as C3A.3(CaSO 4 ).32(H 2 O). Ettringite grows as columns of calcium, aluminum and oxygen surrounded by water and sulfate ions, as shown in [link] .

Tetracalcium aluminoferrite (C4AF) reacts much like C3A, i.e., forming ettringite in the presence of gypsum. However, hydration the ferrite phase is much slower than hydration of C3A, and water is observed to bead up on the surface of C4AF particles. This may be due to the fact that iron is not as free to migrate in the pastes as aluminum, which may cause the formation of a less permeable iron rich layer at the surface of the C4AF particles and isolated regions of iron hydroxide. In cement, if there is insufficient gypsum to convert all of the C4AF to ettringite, then an iron-rich gel forms at the surface of the silicate particles which is proposed to slow down their hydration.
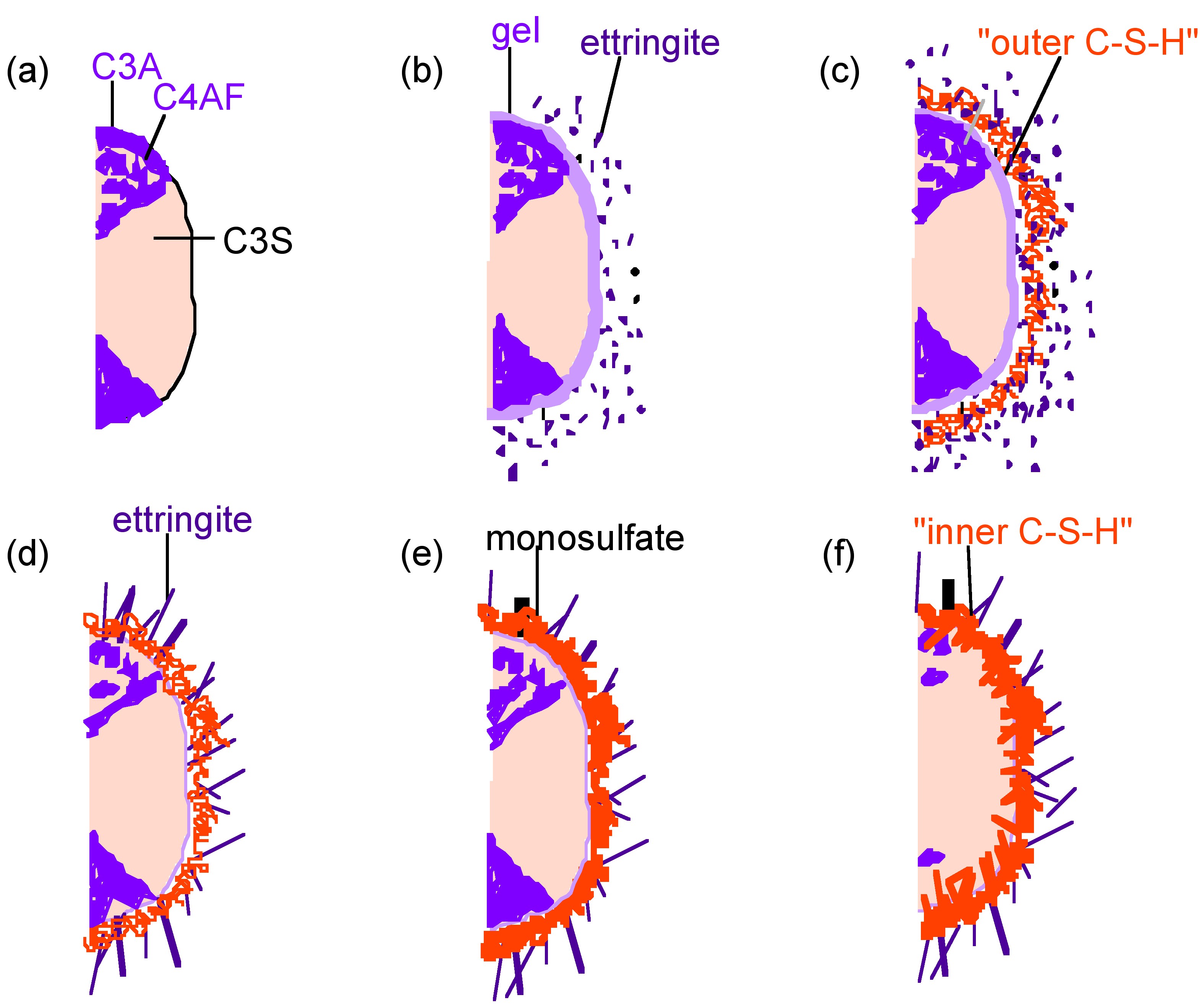
The hydration of cement is obviously far more complex than the sum of the hydration reactions of the individual minerals. The typical depiction of a cement grain involves larger silicate particles surrounded by the much smaller C3A and C4AF particles. The setting (hydration) of cement can be broken down into several distinct periods. The more reactive aluminate and ferrite phases react first, and these reactions dramatically affect the hydration of the silicate phase. Scrivener and Pratt used TEM to develop the widely accepted model depicted in [link] .
In the first few minutes of hydration ( [link] b), the aluminum and iron phases react with gypsum to form an amorphous gel at the surface of the cement grains and short rods of ettringite grow. After this initial period of reactivity, cement hydration slows down and the induction period begins. After about 3 hours of hydration, the induction period ends and the acceleratory period begins. During the period from 3 to 24 hours, about 30% of cement reacts to form calcium hydroxide and C–S–H. The development of C–S–H in this period occurs in 2 phases. After ca. 10 hours hydration ( [link] c), C3S has produced “outer C–S–H,” which grows out from the ettringite rods rather than directly out from the surface of the C3S particles. Therefore, in the initial phase of the reaction, the silicate ions must migrate through the aluminum and iron rich phase to form the C–S–H. In the latter part of the acceleratory period, after 18 hours of hydration, C3A continues to react with gypsum, forming longer ettringite rods ( [link] d). This network of ettringite and C–S–H appears to form a “hydrating shell” about 1 µm from the surface of anhydrous C3S. A small amount of “inner C–S–H” forms inside this shell. After 1–3 days of hydration, reactions slow down and the deceleratory period begins ( [link] e). C3A reacts with ettringite to form some monosulfate. “Inner C–S–H” continues to grow near the C3S surface, narrowing the 1 µm gap between the “hydrating shell” and anhydrous C3S. The rate of hydration is likely to depend on the diffusion rate of water or ions to the anhydrous surface. After 2 weeks hydration ( [link] f), the gap between the “hydrating shell” and the grain is completely filled with C–S–H. The original, “outer C–S–H” becomes more fibrous.

Notification Switch
Would you like to follow the 'Portland cement in the energy industry' conversation and receive update notifications?