| << Chapter < Page | Chapter >> Page > |

Look at each of the processes shown, and decide if it is endergonic or exergonic. In each case, does enthalpy increase or decrease, and does entropy increase or decrease?
An important concept in the study of metabolism and energy is that of chemical equilibrium. Most chemical reactions are reversible. They can proceed in both directions, releasing energy into their environment in one direction, and absorbing it from the environment in the other direction ( [link] ). The same is true for the chemical reactions involved in cell metabolism, such as the breaking down and building up of proteins into and from individual amino acids, respectively. Reactants within a closed system will undergo chemical reactions in both directions until a state of equilibrium is reached. This state of equilibrium is one of the lowest possible free energy and a state of maximal entropy. Energy must be put into the system to push the reactants and products away from a state of equilibrium. Either reactants or products must be added, removed, or changed. If a cell were a closed system, its chemical reactions would reach equilibrium, and it would die because there would be insufficient free energy left to perform the work needed to maintain life. In a living cell, chemical reactions are constantly moving towards equilibrium, but never reach it. This is because a living cell is an open system. Materials pass in and out, the cell recycles the products of certain chemical reactions into other reactions, and chemical equilibrium is never reached. In this way, living organisms are in a constant energy-requiring, uphill battle against equilibrium and entropy. This constant supply of energy ultimately comes from sunlight, which is used to produce nutrients in the process of photosynthesis.
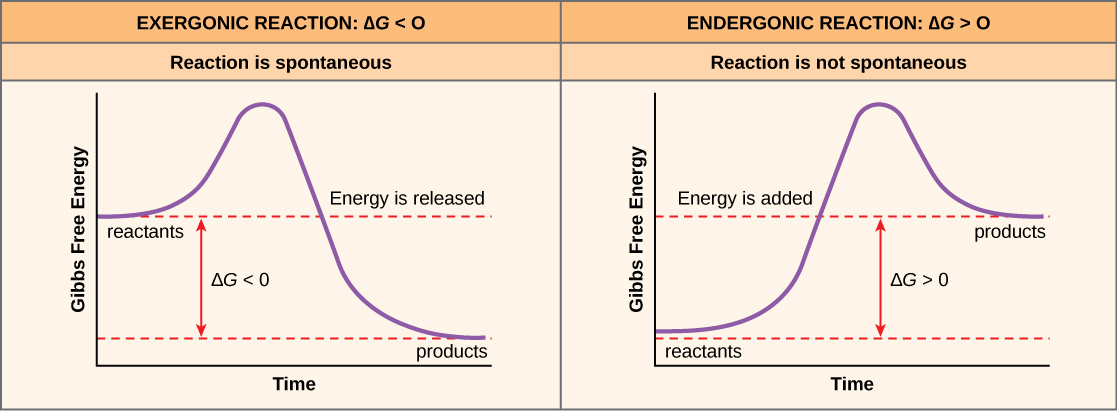
Use figure 4 above. In exergonic reactions, the products have:
b
Use figure 4 above. The information in the deltaG of a reaction:
i
There is another important concept that must be considered regarding endergonic and exergonic reactions. Even exergonic reactions require a small amount of energy input to get going before they can proceed with their energy-releasing steps. These reactions have a net release of energy, but still require some energy in the beginning. This small amount of energy input necessary for all chemical reactions to occur is called the activation energy (or free energy of activation) and is abbreviated E A ( [link] ).

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?