| << Chapter < Page | Chapter >> Page > |
Many photosynthetic organisms have a mixture of pigments; using them, the organism can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through, because the taller trees absorb most of the sunlight and scatter the remaining solar radiation ( [link] ).

When studying a photosynthetic organism, scientists can determine the types of pigments present by generating absorption spectra. An instrument called a spectrophotometer can differentiate which wavelengths of light a substance can absorb. Spectrophotometers measure transmitted light and compute from it the absorption. By extracting pigments from leaves and placing these samples into a spectrophotometer, scientists can identify which wavelengths of light an organism can absorb. Additional methods for the identification of plant pigments include various types of chromatography that separate the pigments by their relative affinities to solid and mobile phases.
The overall function of light-dependent reactions is to convert solar energy into chemical energy in the form of NADPH and ATP. This chemical energy supports the light-independent reactions and fuels the assembly of sugar molecules. The light-dependent reactions are depicted in [link] . Protein complexes and pigment molecules work together to produce NADPH and ATP.
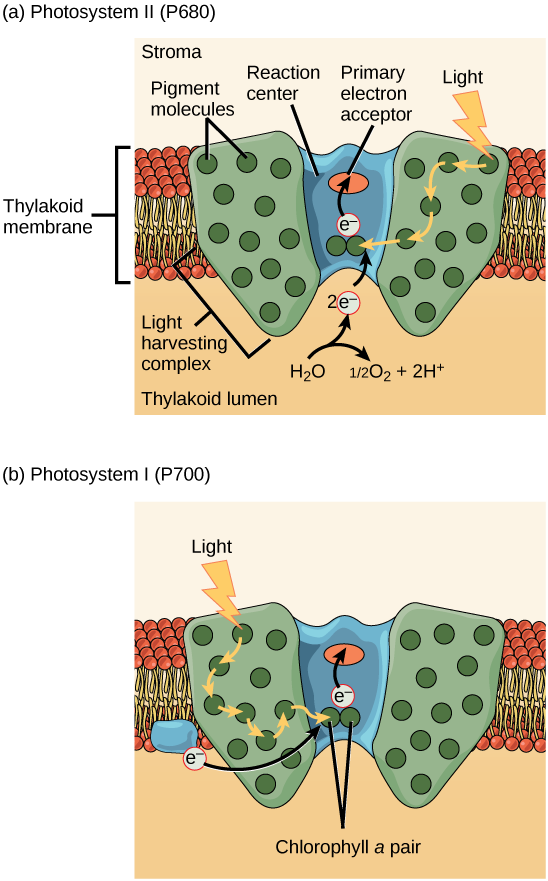
The actual step that converts light energy into chemical energy takes place in a multiprotein complex called a photosystem , two types of which are found embedded in the thylakoid membrane, photosystem II (PSII) and photosystem I (PSI) ( [link] ). The two complexes differ on the basis of what they oxidize (that is, the source of the low-energy electron supply) and what they reduce (the place to which they deliver their energized electrons).
Both photosystems have the same basic structure; a number of antenna proteins to which the chlorophyll molecules are bound surround the reaction center where the photochemistry takes place. Each photosystem is serviced by the light-harvesting complex , which passes energy from sunlight to the reaction center; it consists of multiple antenna proteins that contain a mixture of 300–400 chlorophyll a and b molecules as well as other pigments like carotenoids. The absorption of a single photon or distinct quantity or “packet” of light by any of the chlorophylls pushes that molecule into an excited state. In short, the light energy has now been captured by biological molecules but is not stored in any useful form yet. The energy is transferred from chlorophyll to chlorophyll until eventually (after about a millionth of a second), it is delivered to the reaction center. Up to this point, only energy has been transferred between molecules, not electrons.

Notification Switch
Would you like to follow the 'General biology part i - mixed majors' conversation and receive update notifications?