| << Chapter < Page | Chapter >> Page > |
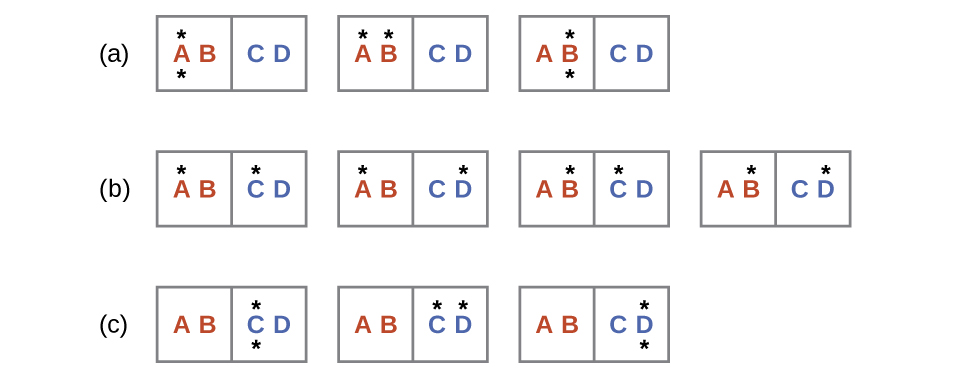

The initial number of microstates is one, the final six:
The sign of this result is consistent with expectation; since there are more microstates possible for the final state than for the initial state, the change in entropy should be positive.
0 J/K
The relationships between entropy, microstates, and matter/energy dispersal described previously allow us to make generalizations regarding the relative entropies of substances and to predict the sign of entropy changes for chemical and physical processes. Consider the phase changes illustrated in [link] . In the solid phase, the atoms or molecules are restricted to nearly fixed positions with respect to each other and are capable of only modest oscillations about these positions. With essentially fixed locations for the system’s component particles, the number of microstates is relatively small. In the liquid phase, the atoms or molecules are free to move over and around each other, though they remain in relatively close proximity to one another. This increased freedom of motion results in a greater variation in possible particle locations, so the number of microstates is correspondingly greater than for the solid. As a result, S liquid > S solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, Δ S >0. By the same logic, the reciprocal process (freezing) exhibits a decrease in entropy, Δ S <0.
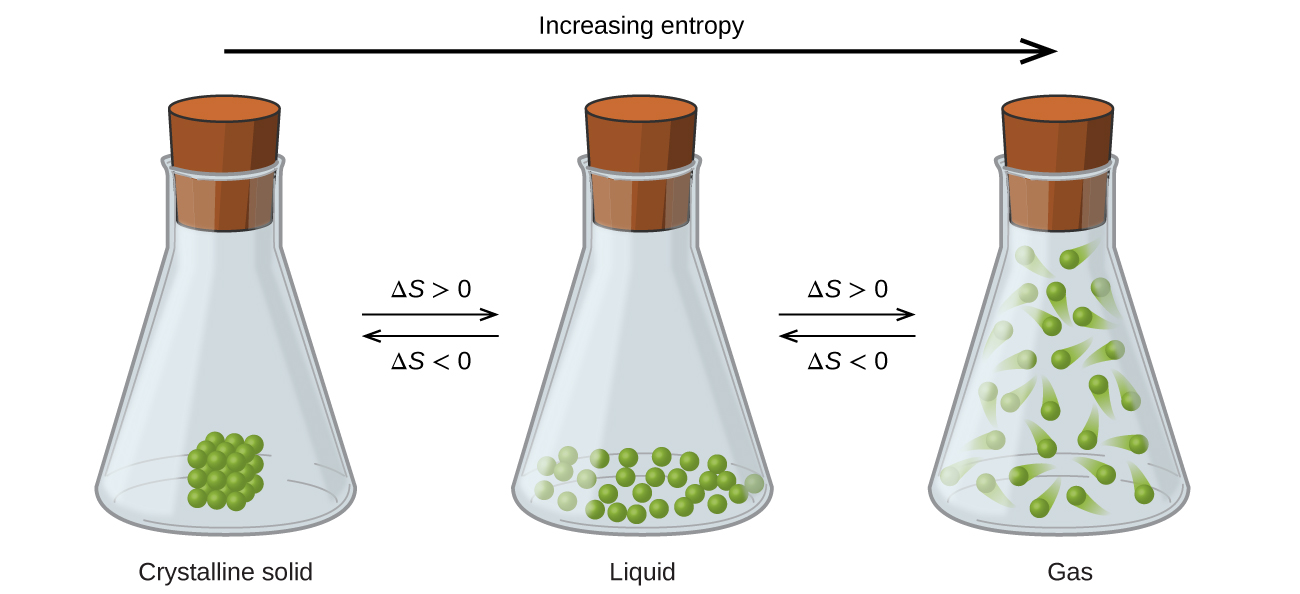
Now consider the vapor or gas phase. The atoms or molecules occupy a much greater volume than in the liquid phase; therefore each atom or molecule can be found in many more locations than in the liquid (or solid) phase. Consequently, for any substance, S gas > S liquid > S solid , and the processes of vaporization and sublimation likewise involve increases in entropy, Δ S >0. Likewise, the reciprocal phase transitions, condensation and deposition, involve decreases in entropy, Δ S <0.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?