| << Chapter < Page | Chapter >> Page > |
Determine the direction of change. The complex ion is in equilibrium with its components, as represented by the equation:
We write the equilibrium as a formation reaction because Appendix K lists formation constants for complex ions. Before equilibrium, the reaction quotient is larger than the equilibrium constant [ K f = 1.7 10 7 , and it is infinitely large], so the reaction shifts to the left to reach equilibrium.
Determine x and equilibrium concentrations. We let the change in concentration of Ag + be x . Dissociation of 1 mol of gives 1 mol of Ag + and 2 mol of NH 3 , so the change in [NH 3 ] is 2 x and that of is – x . In summary:
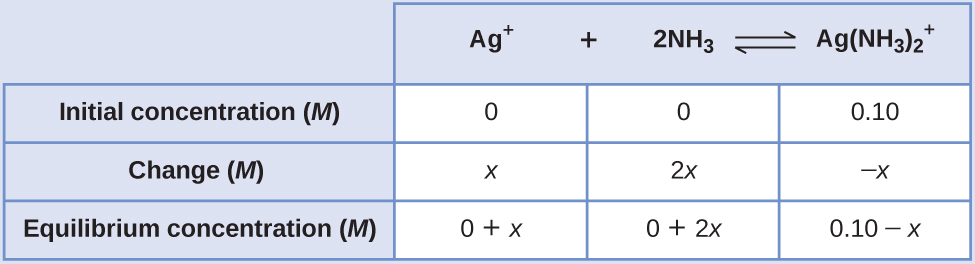
Solve for x and the equilibrium concentrations. At equilibrium:
Both Q and K f are much larger than 1, so let us assume that the changes in concentrations needed to reach equilibrium are small. Thus 0.10 – x is approximated as 0.10:
Because only 1.1% of the dissociates into Ag + and NH 3 , the assumption that x is small is justified.
Now we determine the equilibrium concentrations:
The concentration of free silver ion in the solution is 0.0011 M .
Check the work. The value of Q calculated using the equilibrium concentrations is equal to K f within the error associated with the significant figures in the calculation.
2.5 10 –22 M
G.N. Lewis proposed a definition for acids and bases that relies on an atom’s or molecule’s ability to accept or donate electron pairs. A Lewis acid is a species that can accept an electron pair, whereas a Lewis base has an electron pair available for donation to a Lewis acid. Complex ions are examples of Lewis acid-base adducts. In a complex ion, we have a central atom, often consisting of a transition metal cation, which acts as a Lewis acid, and several neutral molecules or ions surrounding them called ligands that act as Lewis bases. Complex ions form by sharing electron pairs to form coordinate covalent bonds. The equilibrium reaction that occurs when forming a complex ion has an equilibrium constant associated with it called a formation constant, K f . This is often referred to as a stability constant, as it represents the stability of the complex ion. Formation of complex ions in solution can have a profound effect on the solubility of a transition metal compound.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?