| << Chapter < Page | Chapter >> Page > |
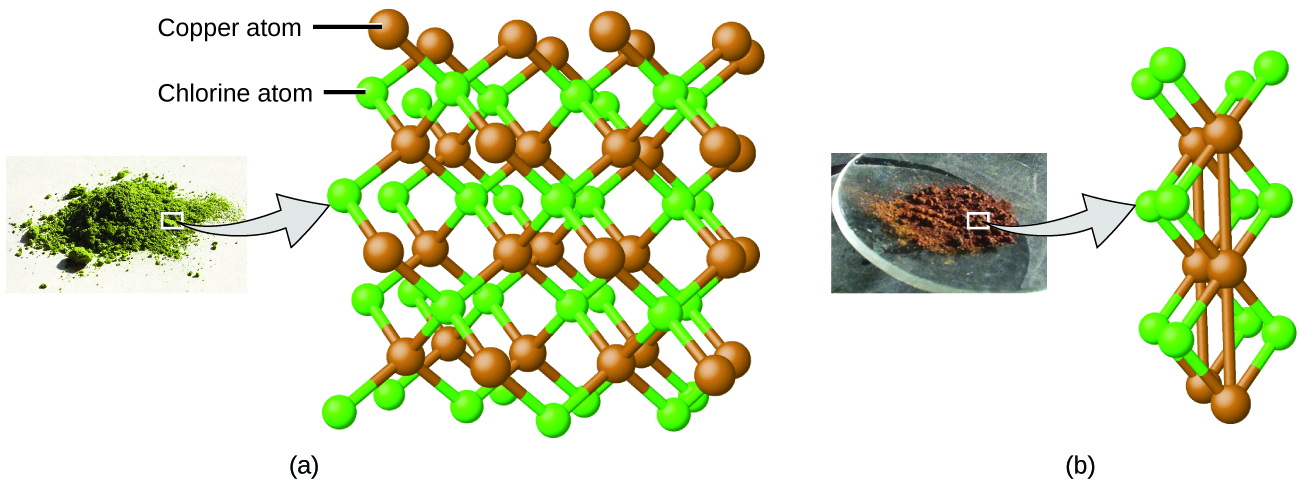
Dalton also used data from Proust, as well as results from his own experiments, to formulate another interesting law. The law of multiple proportions states that when two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small, whole numbers . For example, copper and chlorine can form a green, crystalline solid with a mass ratio of 0.558 g chlorine to 1 g copper, as well as a brown crystalline solid with a mass ratio of 1.116 g chlorine to 1 g copper. These ratios by themselves may not seem particularly interesting or informative; however, if we take a ratio of these ratios, we obtain a useful and possibly surprising result: a small, whole-number ratio.
This 2-to-1 ratio means that the brown compound has twice the amount of chlorine per amount of copper as the green compound.
This can be explained by atomic theory if the copper-to-chlorine ratio in the brown compound is 1 copper atom to 2 chlorine atoms, and the ratio in the green compound is 1 copper atom to 1 chlorine atom. The ratio of chlorine atoms (and thus the ratio of their masses) is therefore 2 to 1 ( [link] ).
In compound B, the mass ratio of carbon to oxygen is:
The ratio of these ratios is:
This supports the law of multiple proportions. This means that A and B are different compounds, with A having one-half as much carbon per amount of oxygen (or twice as much oxygen per amount of carbon) as B. A possible pair of compounds that would fit this relationship would be A = CO 2 and B = CO.
In compound X, the mass ratio of carbon to hydrogen is In compound Y, the mass ratio of carbon to oxygen is The ratio of these ratios is This small, whole-number ratio supports the law of multiple proportions. This means that X and Y are different compounds.
The ancient Greeks proposed that matter consists of extremely small particles called atoms. Dalton postulated that each element has a characteristic type of atom that differs in properties from atoms of all other elements, and that atoms of different elements can combine in fixed, small, whole-number ratios to form compounds. Samples of a particular compound all have the same elemental proportions by mass. When two elements form different compounds, a given mass of one element will combine with masses of the other element in a small, whole-number ratio. During any chemical change, atoms are neither created nor destroyed.
In the following drawing, the green spheres represent atoms of a certain element. The purple spheres represent atoms of another element. If the spheres of different elements touch, they are part of a single unit of a compound. The following chemical change represented by these spheres may violate one of the ideas of Dalton’s atomic theory. Which one?
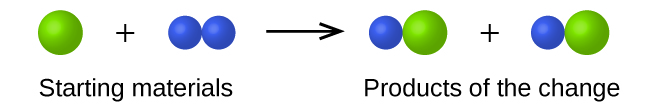
The starting materials consist of one green sphere and two purple spheres. The products consist of two green spheres and two purple spheres. This violates Dalton’s postulate that that atoms are not created during a chemical change, but are merely redistributed.
Which postulate of Dalton’s theory is consistent with the following observation concerning the weights of reactants and products? When 100 grams of solid calcium carbonate is heated, 44 grams of carbon dioxide and 56 grams of calcium oxide are produced.
Identify the postulate of Dalton’s theory that is violated by the following observations: 59.95% of one sample of titanium dioxide is titanium; 60.10% of a different sample of titanium dioxide is titanium.
This statement violates Dalton’s fourth postulate: In a given compound, the numbers of atoms of each type (and thus also the percentage) always have the same ratio.
Samples of compound X, Y, and Z are analyzed, with results shown here.
| Compound | Description | Mass of Carbon | Mass of Hydrogen |
|---|---|---|---|
| X | clear, colorless, liquid with strong odor | 1.776 g | 0.148 g |
| Y | clear, colorless, liquid with strong odor | 1.974 g | 0.329 g |
| Z | clear, colorless, liquid with strong odor | 7.812 g | 0.651 g |
Do these data provide example(s) of the law of definite proportions, the law of multiple proportions, neither, or both? What do these data tell you about compounds X, Y, and Z?

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?