| << Chapter < Page | Chapter >> Page > |
As all substances must be electrically neutral, the total number of positive charges on the cations of an ionic compound must equal the total number of negative charges on its anions. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative charges. For example, the formula for aluminum oxide, Al 2 O 3 , indicates that this ionic compound contains two aluminum cations, Al 3+ , for every three oxide anions, O 2− [thus, (2 +3) + (3 –2) = 0].
It is important to note, however, that the formula for an ionic compound does not represent the physical arrangement of its ions. It is incorrect to refer to a sodium chloride (NaCl) “molecule” because there is not a single ionic bond, per se, between any specific pair of sodium and chloride ions. The attractive forces between ions are isotropic—the same in all directions—meaning that any particular ion is equally attracted to all of the nearby ions of opposite charge. This results in the ions arranging themselves into a tightly bound, three-dimensional lattice structure. Sodium chloride, for example, consists of a regular arrangement of equal numbers of Na + cations and Cl – anions ( [link] ).
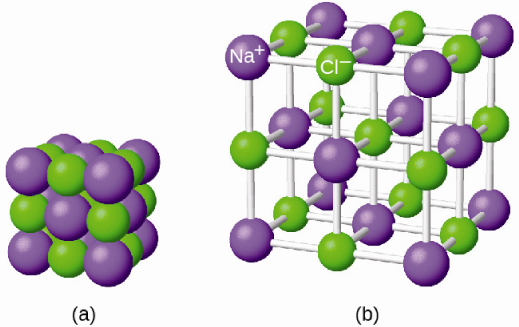
The strong electrostatic attraction between Na + and Cl – ions holds them tightly together in solid NaCl. It requires 769 kJ of energy to dissociate one mole of solid NaCl into separate gaseous Na + and Cl – ions:
When forming a cation, an atom of a main group element tends to lose all of its valence electrons, thus assuming the electronic structure of the noble gas that precedes it in the periodic table. For groups 1 (the alkali metals) and 2 (the alkaline earth metals), the group numbers are equal to the numbers of valence shell electrons and, consequently, to the charges of the cations formed from atoms of these elements when all valence shell electrons are removed. For example, calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 . When a Ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 . The Ca 2+ ion is therefore isoelectronic with the noble gas Ar.
For groups 12–17, the group numbers exceed the number of valence electrons by 10 (accounting for the possibility of full d subshells in atoms of elements in the fourth and greater periods). Thus, the charge of a cation formed by the loss of all valence electrons is equal to the group number minus 10. For example, aluminum (in group 13) forms 3+ ions (Al 3+ ).

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?