| << Chapter < Page | Chapter >> Page > |
The manufacture of 683 billion ft 3 of NH 3 required 1020 billion ft 3 of H 2 . (At 25 °C and 1 atm, this is the volume of a cube with an edge length of approximately 1.9 miles.)
51.0 L
The first two conversions are:
Finally, we can use the ideal gas law:
1.30 10 3 L
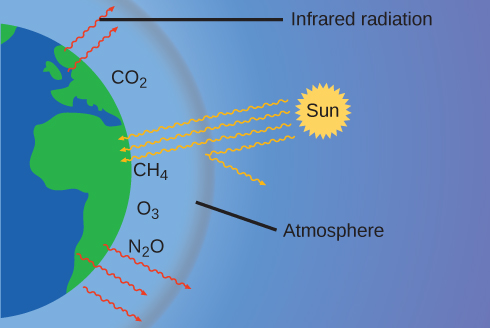
The thin skin of our atmosphere keeps the earth from being an ice planet and makes it habitable. In fact, this is due to less than 0.5% of the air molecules. Of the energy from the sun that reaches the earth, almost is reflected back into space, with the rest absorbed by the atmosphere and the surface of the earth. Some of the energy that the earth absorbs is re-emitted as infrared (IR) radiation, a portion of which passes back out through the atmosphere into space. However, most of this IR radiation is absorbed by certain substances in the atmosphere, known as greenhouse gases, which re-emit this energy in all directions, trapping some of the heat. This maintains favorable living conditions—without atmosphere, the average global average temperature of 14 °C (57 °F) would be about –19 °C (–2 °F). The major greenhouse gases (GHGs) are water vapor, carbon dioxide, methane, and ozone. Since the Industrial Revolution, human activity has been increasing the concentrations of GHGs, which have changed the energy balance and are significantly altering the earth’s climate ( [link] ).
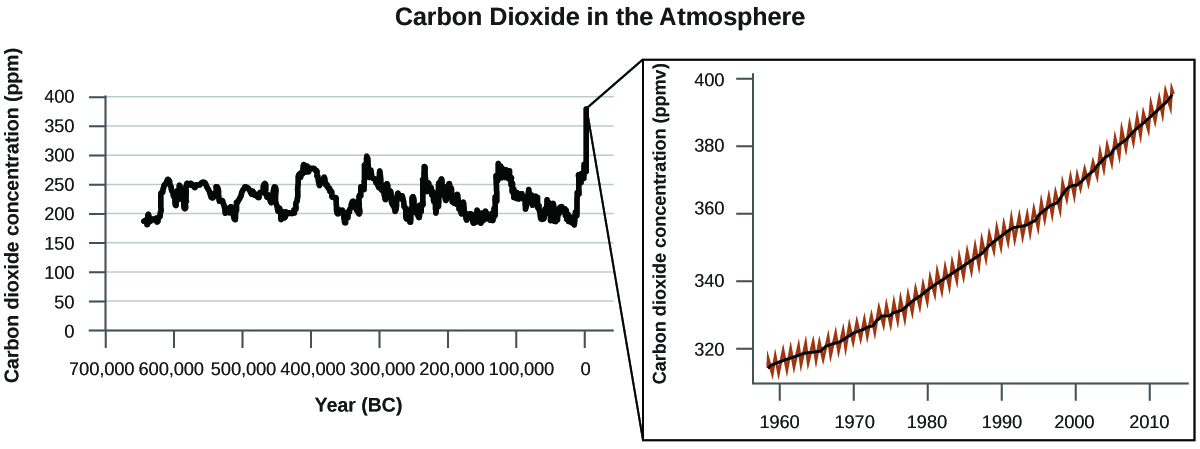
There is strong evidence from multiple sources that higher atmospheric levels of CO 2 are caused by human activity, with fossil fuel burning accounting for about of the recent increase in CO 2 . Reliable data from ice cores reveals that CO 2 concentration in the atmosphere is at the highest level in the past 800,000 years; other evidence indicates that it may be at its highest level in 20 million years. In recent years, the CO 2 concentration has increased from historical levels of below 300 ppm to almost 400 ppm today ( [link] ).

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?