| << Chapter < Page | Chapter >> Page > |
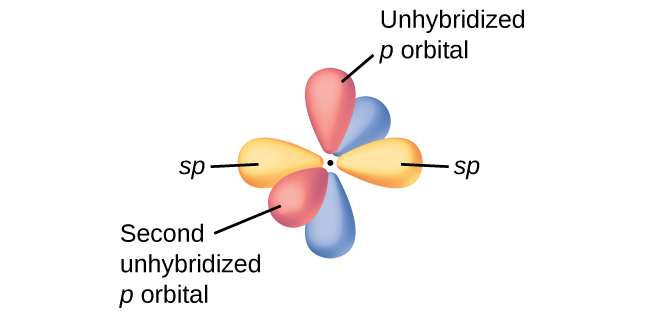
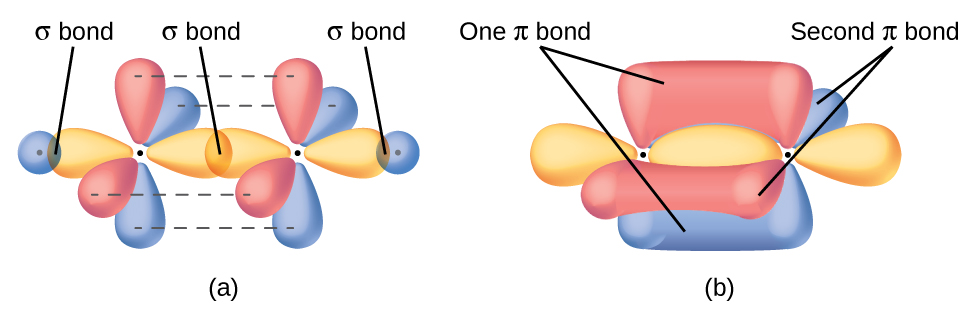
In molecules with sp hybrid orbitals, two unhybridized p orbitals remain on the atom ( [link] ). We find this situation in acetylene, which is a linear molecule. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between the carbon atoms ( [link] ). The remaining sp orbitals form σ bonds with hydrogen atoms. The two unhybridized p orbitals per carbon are positioned such that they overlap side by side and, hence, form two π bonds. The two carbon atoms of acetylene are thus bound together by one σ bond and two π bonds, giving a triple bond.
Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired electrons (radicals). Structures that account for these features describe the correct hybridization of the atoms. However, many structures also include resonance forms. Remember that resonance forms occur when various arrangements of π bonds are possible. Since the arrangement of π bonds involves only the unhybridized orbitals, resonance does not influence the assignment of hybridization.
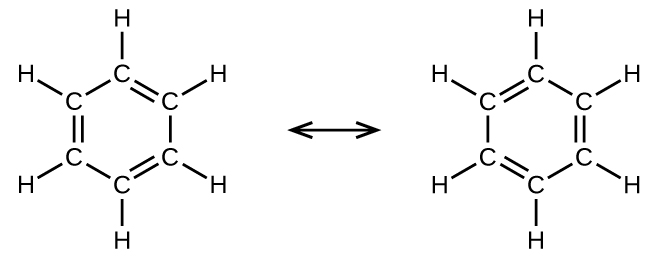
For example, molecule benzene has two resonance forms ( [link] ). We can use either of these forms to determine that each of the carbon atoms is bonded to three other atoms with no lone pairs, so the correct hybridization is sp 2 . The electrons in the unhybridized p orbitals form π bonds. Neither resonance structure completely describes the electrons in the π bonds. They are not located in one position or the other, but in reality are delocalized throughout the ring. Valence bond theory does not easily address delocalization. Bonding in molecules with resonance forms is better described by molecular orbital theory. (See the next module.)
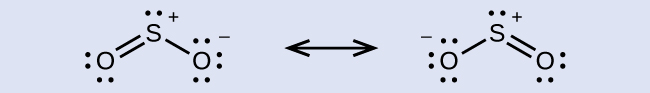
The sulfur atom is surrounded by two bonds and one lone pair of electrons in either resonance structure. Therefore, the electron-pair geometry is trigonal planar, and the hybridization of the sulfur atom is sp 2 .
sp 2
Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. The σ bonds are usually formed by the overlap of hybridized atomic orbitals, while the π bonds are formed by the side-by-side overlap of unhybridized orbitals. Resonance occurs when there are multiple unhybridized orbitals with the appropriate alignment to overlap, so the placement of π bonds can vary.
The bond energy of a C–C single bond averages 347 kJ mol −1 ; that of a triple bond averages 839 kJ mol −1 . Explain why the triple bond is not three times as strong as a single bond.
A triple bond consists of one σ bond and two π bonds. A σ bond is stronger than a π bond due to greater overlap.
For the carbonate ion, draw all of the resonance structures. Identify which orbitals overlap to create each bond.
A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H 3 CCN. It is present in paint strippers.
(a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
(c) Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.
(a)
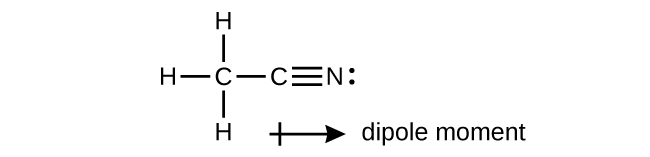
(b) The terminal carbon atom uses
sp
3 hybrid orbitals, while the central carbon atom is
sp hybridized. (c) Each of the two π bonds is formed by overlap of a 2
p orbital on carbon and a nitrogen 2
p orbital.
For the molecule allene, give the hybridization of each carbon atom. Will the hydrogen atoms be in the same plane or perpendicular planes?
Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
(a) ClNO (N is the central atom)
(b) CS 2
(c) Cl 2 CO (C is the central atom)
(d) Cl 2 SO (S is the central atom)
(e) SO 2 F 2 (S is the central atom)
(f) XeO 2 F 2 (Xe is the central atom)
(g) (Cl is the central atom)
(a) sp 2 ; (b) sp ; (c) sp 2 ; (d) sp 3 ; (e) sp 3 ; (f) sp 3 d ; (g) sp 3
Describe the molecular geometry and hybridization of the N, P, or S atoms in each of the following compounds.
(a) H 3 PO 4 , phosphoric acid, used in cola soft drinks
(b) NH 4 NO 3 , ammonium nitrate, a fertilizer and explosive
(c) S 2 Cl 2 , disulfur dichloride, used in vulcanizing rubber
(d) K 4 [O 3 POPO 3 ], potassium pyrophosphate, an ingredient in some toothpastes
For each of the following molecules, indicate the hybridization requested and whether or not the electrons will be delocalized:
(a) ozone (O 3 ) central O hybridization
(b) carbon dioxide (CO 2 ) central C hybridization
(c) nitrogen dioxide (NO 2 ) central N hybridization
(d) phosphate ion central P hybridization
(a) sp 2 , delocalized; (b) sp , localized; (c) sp 2 , delocalized; (d) sp 3 , delocalized
For each of the following structures, determine the hybridization requested and whether the electrons will be delocalized:
(a) Hybridization of each carbon
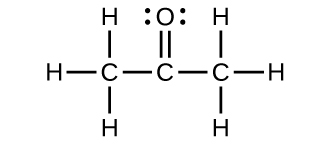
(b) Hybridization of sulfur
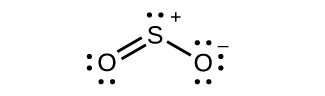
(c) All atoms
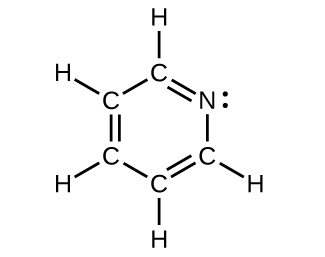
Draw the orbital diagram for carbon in CO 2 showing how many carbon atom electrons are in each orbital.
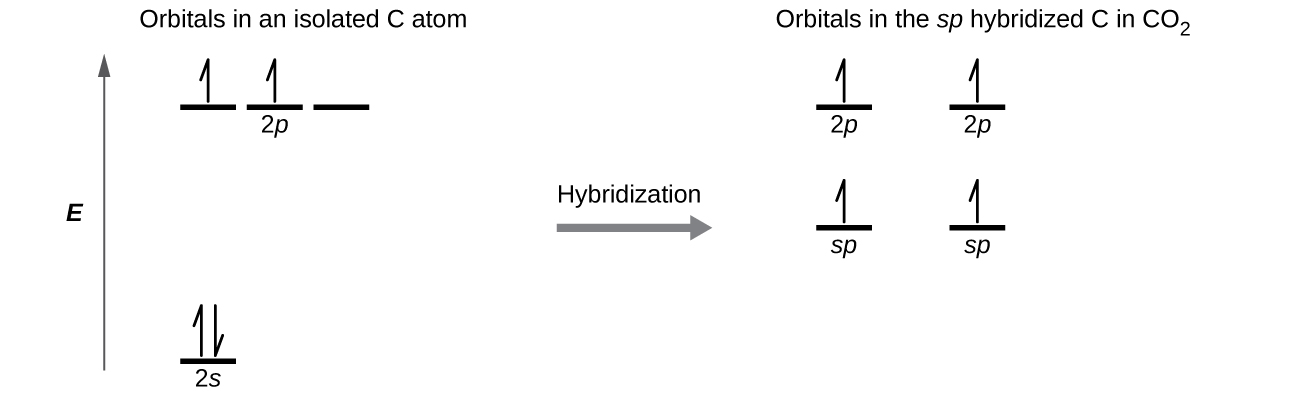
Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?