| << Chapter < Page | Chapter >> Page > |
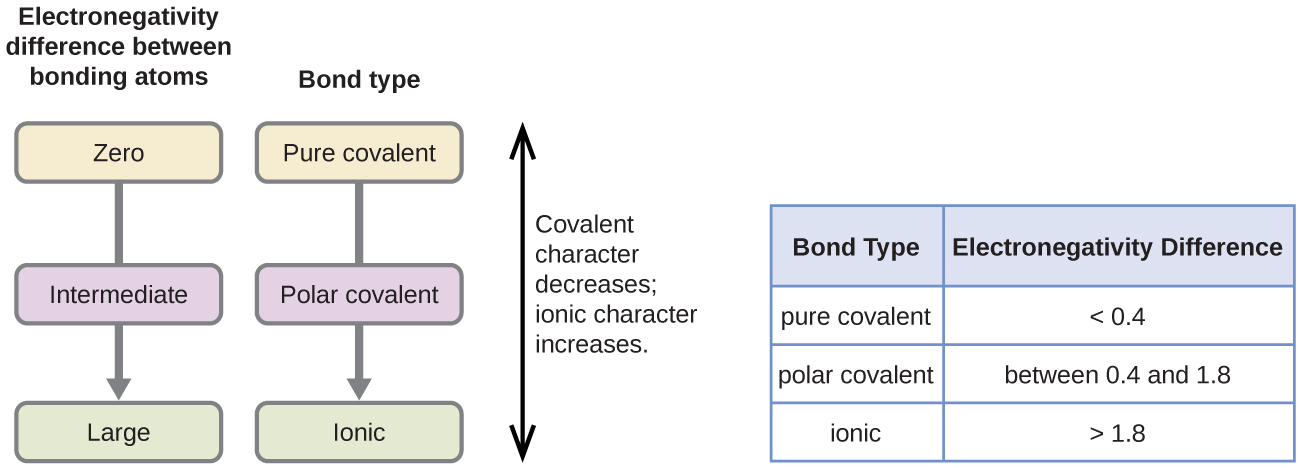
A rough approximation of the electronegativity differences associated with covalent, polar covalent, and ionic bonds is shown in [link] . This table is just a general guide, however, with many exceptions. For example, the H and F atoms in HF have an electronegativity difference of 1.9, and the N and H atoms in NH 3 a difference of 0.9, yet both of these compounds form bonds that are considered polar covalent. Likewise, the Na and Cl atoms in NaCl have an electronegativity difference of 2.1, and the Mn and I atoms in MnI 2 have a difference of 1.0, yet both of these substances form ionic compounds.
The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often ionic.
Some compounds contain both covalent and ionic bonds. The atoms in polyatomic ions, such as OH – , and are held together by polar covalent bonds. However, these polyatomic ions form ionic compounds by combining with ions of opposite charge. For example, potassium nitrate, KNO 3 , contains the K + cation and the polyatomic anion. Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic attraction between the ions K + and as well as covalent between the nitrogen and oxygen atoms in
C–H, C–N, C–O, N–H, O–H, S–H
| Bond Polarity and Electronegativity Difference | ||
|---|---|---|
| Bond | ΔEN | Polarity |
| C–H | 0.4 | |
| S–H | 0.4 | |
| C–N | 0.5 | |
| N–H | 0.9 | |
| C–O | 1.0 | |
| O–H | 1.4 |
| Bond | Electronegativity Difference | Polarity |
|---|---|---|
| C–C | 0.0 | nonpolar |
| C–H | 0.4 | |
| Si–C | 0.7 | |
| Si–O | 1.7 |
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. In pure covalent bonds, the electrons are shared equally. In polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the electrons than the other. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. The difference in electronegativity between two atoms determines how polar a bond will be. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic.
Why is it incorrect to speak of a molecule of solid NaCl?
NaCl consists of discrete ions arranged in a crystal lattice, not covalently bonded molecules.
What information can you use to predict whether a bond between two atoms is covalent or ionic?
Predict which of the following compounds are ionic and which are covalent, based on the location of their constituent atoms in the periodic table:
(a) Cl 2 CO
(b) MnO
(c) NCl 3
(d) CoBr 2
(e) K 2 S
(f) CO
(g) CaF 2
(h) HI
(i) CaO
(j) IBr
(k) CO 2
ionic: (b), (d), (e), (g), and (i); covalent: (a), (c), (f), (h), (j), and (k)
Explain the difference between a nonpolar covalent bond, a polar covalent bond, and an ionic bond.
From its position in the periodic table, determine which atom in each pair is more electronegative:
(a) Br or Cl
(b) N or O
(c) S or O
(d) P or S
(e) Si or N
(f) Ba or P
(g) N or K
(a) Cl; (b) O; (c) O; (d) S; (e) N; (f) P; (g) N
From its position in the periodic table, determine which atom in each pair is more electronegative:
(a) N or P
(b) N or Ge
(c) S or F
(d) Cl or S
(e) H or C
(f) Se or P
(g) C or Si
From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
(a) C, F, H, N, O
(b) Br, Cl, F, H, I
(c) F, H, O, P, S
(d) Al, H, Na, O, P
(e) Ba, H, N, O, As
(a) H, C, N, O, F; (b) H, I, Br, Cl, F; (c) H, P, S, O, F; (d) Na, Al, H, P, O; (e) Ba, H, As, N, O
From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
(a) As, H, N, P, Sb
(b) Cl, H, P, S, Si
(c) Br, Cl, Ge, H, Sr
(d) Ca, H, K, N, Si
(e) Cl, Cs, Ge, H, Sr
Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?
N, O, F, and Cl
Which is the most polar bond?
(a) C–C
(b) C–H
(c) N–H
(d) O–H
(e) Se–H
Identify the more polar bond in each of the following pairs of bonds:
(a) HF or HCl
(b) NO or CO
(c) SH or OH
(d) PCl or SCl
(e) CH or NH
(f) SO or PO
(g) CN or NN
(a) HF; (b) CO; (c) OH; (d) PCl; (e) NH; (f) PO; (g) CN
Which of the following molecules or ions contain polar bonds?
(a) O 3
(b) S 8
(c)
(d)
(e) CO 2
(f) H 2 S
(g)

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?