| << Chapter < Page | Chapter >> Page > |

Using these data,
The combustion of 1.00 L of isooctane produces 33,100 kJ of heat. (This amount of energy is enough to melt 99.2 kg, or about 218 lbs, of ice.)
Note: If you do this calculation one step at a time, you would find:
6.25 10 3 kJ
As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. Among the most promising biofuels are those derived from algae ( [link] ). The species of algae used are nontoxic, biodegradable, and among the world’s fastest growing organisms. About 50% of algal weight is oil, which can be readily converted into fuel such as biodiesel. Algae can yield 26,000 gallons of biofuel per hectare—much more energy per acre than other crops. Some strains of algae can flourish in brackish water that is not usable for growing other crops. Algae can produce biodiesel, biogasoline, ethanol, butanol, methane, and even jet fuel.
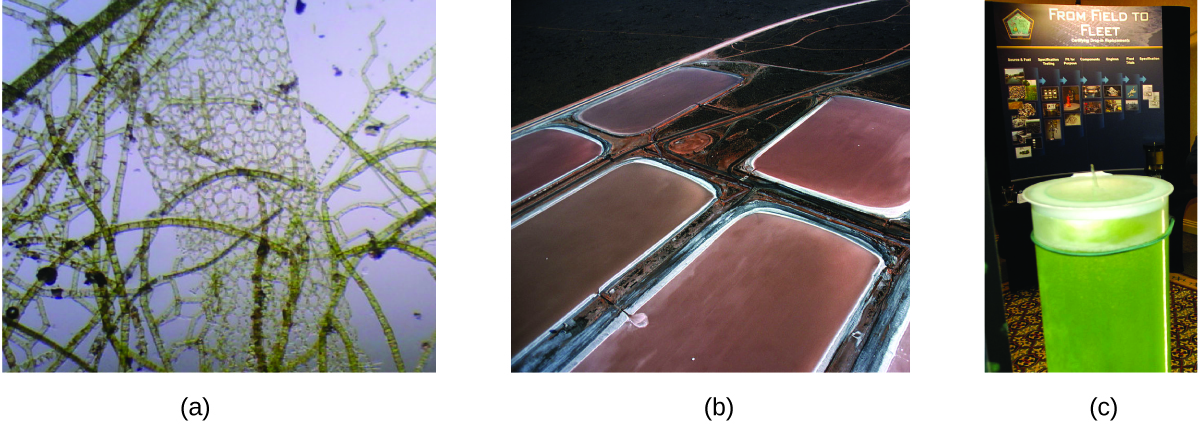
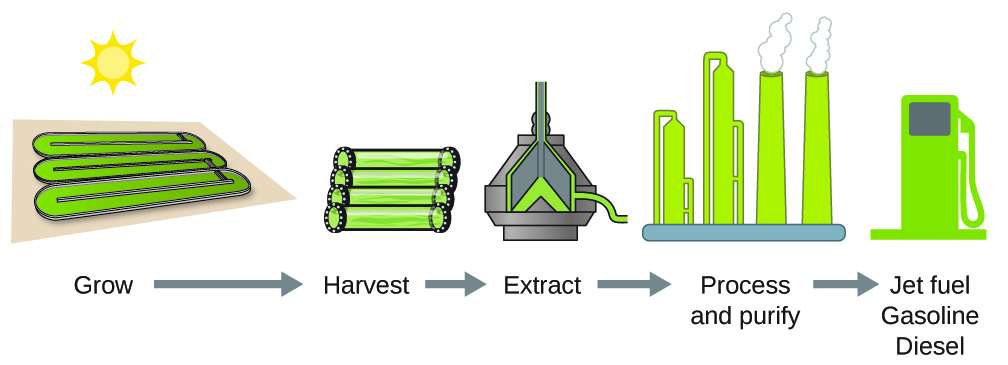
According to the US Department of Energy, only 39,000 square kilometers (about 0.4% of the land mass of the US or less than of the area used to grow corn) can produce enough algal fuel to replace all the petroleum-based fuel used in the US. The cost of algal fuels is becoming more competitive—for instance, the US Air Force is producing jet fuel from algae at a total cost of under $5 per gallon. For more on algal fuel, see http://www.theguardian.com/environment/2010/feb/13/algae-solve-pentagon-fuel-problem. The process used to produce algal fuel is as follows: grow the algae (which use sunlight as their energy source and CO 2 as a raw material); harvest the algae; extract the fuel compounds (or precursor compounds); process as necessary (e.g., perform a transesterification reaction to make biodiesel); purify; and distribute ( [link] ).

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?