| << Chapter < Page | Chapter >> Page > |
The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have determined the number of entities composing 1 mole of substance to be 6.022 10 23 , a quantity called Avogadro’s number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass (amu) and molar mass (g/mol) for any substance are numerically equivalent (for example, one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs approximately 18 g).
What is the total mass (amu) of carbon in each of the following molecules?
(a) CH 4
(b) CHCl 3
(c) C 12 H 10 O 6
(d) CH 3 CH 2 CH 2 CH 2 CH 3
(a) 12.01 amu; (b) 12.01 amu; (c) 144.12 amu; (d) 60.05 amu
What is the total mass of hydrogen in each of the molecules?
(a) CH 4
(b) CHCl 3
(c) C 12 H 10 O 6
(d) CH 3 CH 2 CH 2 CH 2 CH 3
Calculate the molecular or formula mass of each of the following:
(a) P 4
(b) H 2 O
(c) Ca(NO 3 ) 2
(d) CH 3 CO 2 H (acetic acid)
(e) C 12 H 22 O 11 (sucrose, cane sugar).
(a) 123.896 amu; (b) 18.015 amu; (c) 164.086 amu; (d) 60.052 amu; (e) 342.297 amu
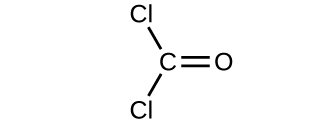
Determine the molecular mass of the following compounds:
(a)
(b)
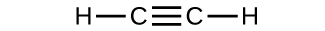
(c)
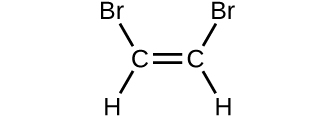
(d)
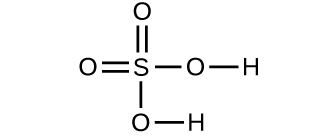
Determine the molecular mass of the following compounds:
(a)
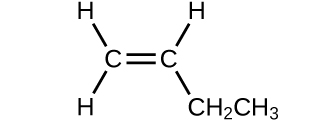
(b)
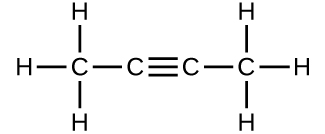
(c)
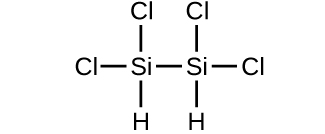
(d)
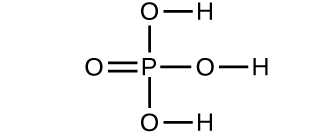
(a) 56.107 amu;
(b) 54.091 amu;
(c) 199.9976 amu;
(d) 97.9950 amu
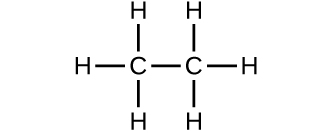
Which molecule has a molecular mass of 28.05 amu?
(a)
![]()
(b)
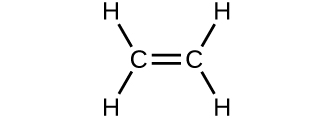
(c)
Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula.
Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams.
Compare 1 mole of H 2 , 1 mole of O 2 , and 1 mole of F 2 .
(a) Which has the largest number of molecules? Explain why.
(b) Which has the greatest mass? Explain why.
Which contains the greatest mass of oxygen: 0.75 mol of ethanol (C 2 H 5 OH), 0.60 mol of formic acid (HCO 2 H), or 1.0 mol of water (H 2 O)? Explain why.
Formic acid. Its formula has twice as many oxygen atoms as the other two compounds (one each). Therefore, 0.60 mol of formic acid would be equivalent to 1.20 mol of a compound containing a single oxygen atom.
Which contains the greatest number of moles of oxygen atoms: 1 mol of ethanol (C 2 H 5 OH), 1 mol of formic acid (HCO 2 H), or 1 mol of water (H 2 O)? Explain why.
How are the molecular mass and the molar mass of a compound similar and how are they different?
The two masses have the same numerical value, but the units are different: The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 10 23 molecules.
Calculate the molar mass of each of the following compounds:
(a) hydrogen fluoride, HF
(b) ammonia, NH 3
(c) nitric acid, HNO 3
(d) silver sulfate, Ag 2 SO 4
(e) boric acid, B(OH) 3

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?