| << Chapter < Page | Chapter >> Page > |
Acetic acid, CH 3 CO 2 H, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odor and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
The distinctive and attractive odors and flavors of many flowers, perfumes, and ripe fruits are due to the presence of one or more esters ( [link] ). Among the most important of the natural esters are fats (such as lard, tallow, and butter) and oils (such as linseed, cottonseed, and olive oils), which are esters of the trihydroxyl alcohol glycerine, C 3 H 5 (OH) 3 , with large carboxylic acids, such as palmitic acid, CH 3 (CH 2 ) 14 CO 2 H, stearic acid, CH 3 (CH 2 ) 16 CO 2 H, and oleic acid, Oleic acid is an unsaturated acid; it contains a double bond. Palmitic and stearic acids are saturated acids that contain no double or triple bonds.

Functional groups related to the carbonyl group include the –CHO group of an aldehyde, the –CO– group of a ketone, the –CO 2 H group of a carboxylic acid, and the –CO 2 R group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules: Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
Order the following molecules from least to most oxidized, based on the marked carbon atom:
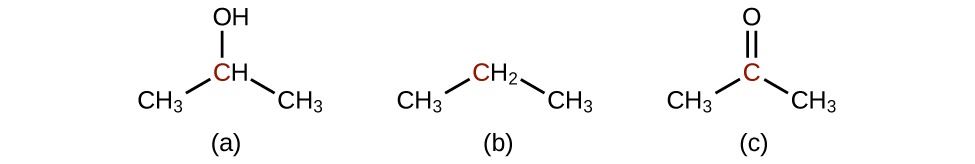
Predict the products of oxidizing the molecules shown in this problem. In each case, identify the product that will result from the minimal increase in oxidation state for the highlighted carbon atom:
(a)
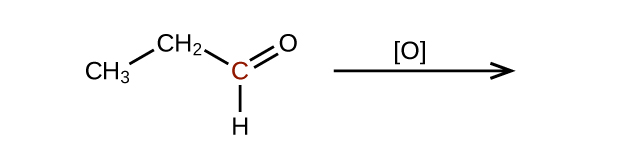
(b)
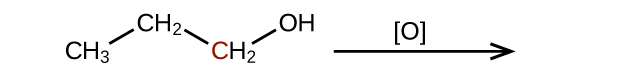
(c)
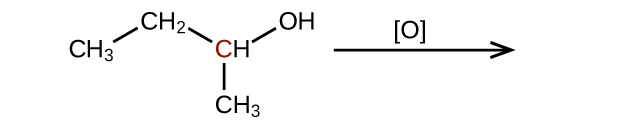
(a)
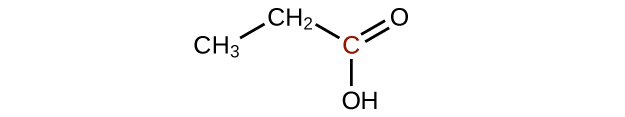 ;
;
(b)
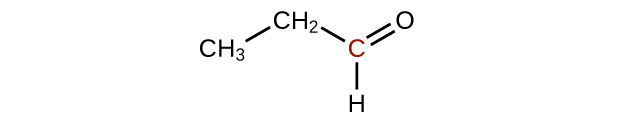 ;
;
(c)
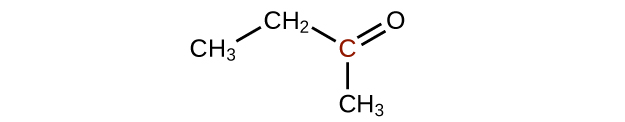
Predict the products of reducing the following molecules. In each case, identify the product that will result from the minimal decrease in oxidation state for the highlighted carbon atom:
(a)
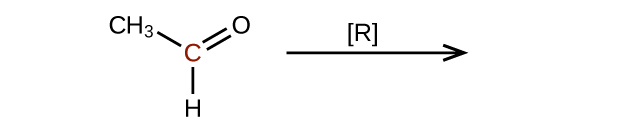
(b)
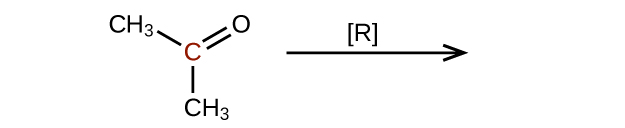
(c)
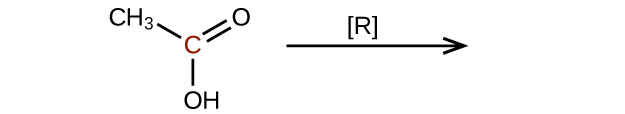
Explain why it is not possible to prepare a ketone that contains only two carbon atoms.
A ketone contains a group bonded to two additional carbon atoms; thus, a minimum of three carbon atoms are needed.
How does hybridization of the substituted carbon atom change when an alcohol is converted into an aldehyde? An aldehyde to a carboxylic acid?
Fatty acids are carboxylic acids that have long hydrocarbon chains attached to a carboxylate group. How does a saturated fatty acid differ from an unsaturated fatty acid? How are they similar?
Since they are both carboxylic acids, they each contain the –COOH functional group and its characteristics. The difference is the hydrocarbon chain in a saturated fatty acid contains no double or triple bonds, whereas the hydrocarbon chain in an unsaturated fatty acid contains one or more multiple bonds.
Write a condensed structural formula, such as CH 3 CH 3 , and describe the molecular geometry at each carbon atom.
(a) propene
(b) 1-butanol
(c) ethyl propyl ether
(d) cis -4-bromo-2-heptene
(e) 2,2,3-trimethylhexane
(f) formaldehyde
Write a condensed structural formula, such as CH 3 CH 3 , and describe the molecular geometry at each carbon atom.
(a) 2-propanol
(b) acetone
(c) dimethyl ether
(d) acetic acid
(e) 3-methyl-1-hexene
(a) CH 3 CH(OH)CH 3 : all carbons are tetrahedral; (b) the end carbons are tetrahedral and the central carbon is trigonal planar; (c) CH 3 OCH 3 : all are tetrahedral; (d) CH 3 COOH: the methyl carbon is tetrahedral and the acid carbon is trigonal planar; (e) CH 3 CH 2 CH 2 CH(CH 3 )CHCH 2 : all are tetrahedral except the right-most two carbons, which are trigonal planar
The foul odor of rancid butter is caused by butyric acid, CH 3 CH 2 CH 2 CO 2 H.
(a) Draw the Lewis structure and determine the oxidation number and hybridization for each carbon atom in the molecule.
(b) The esters formed from butyric acid are pleasant-smelling compounds found in fruits and used in perfumes. Draw the Lewis structure for the ester formed from the reaction of butyric acid with 2-propanol.
Write two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures:
(a) ethanol reacts with propionic acid
(b) benzoic acid, C 6 H 5 CO 2 H, is added to a solution of sodium hydroxide
Write two complete balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.
(a) 1-butanol reacts with acetic acid
(b) propionic acid is poured onto solid calcium carbonate
(a)
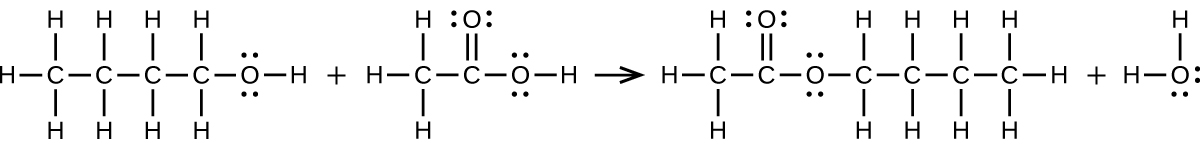 ;
;
(b)
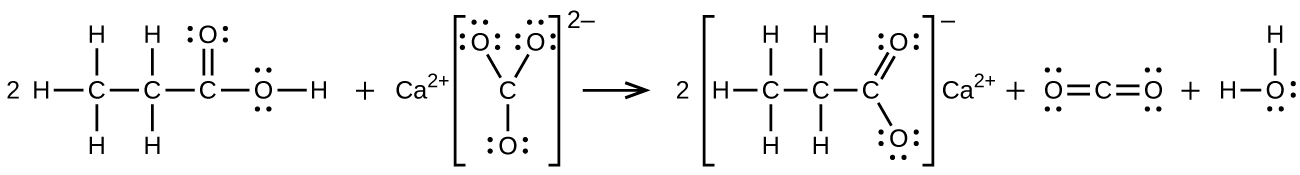
Yields in organic reactions are sometimes low. What is the percent yield of a process that produces 13.0 g of ethyl acetate from 10.0 g of CH 3 CO 2 H?
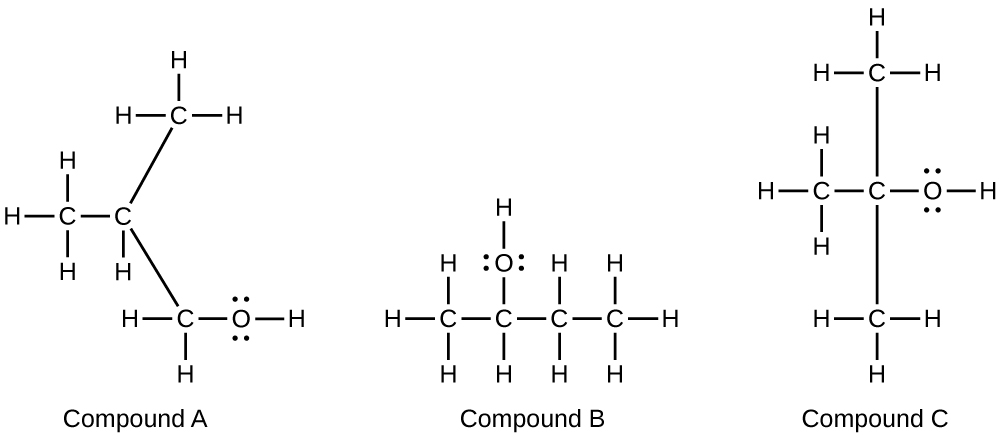
Alcohols A, B, and C all have the composition C 4 H 10 O. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidized to a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?